Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO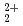 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Lothenbach, B.*; Ochs, M.*; Wanner, H.*; Yui, Mikazu
JNC TN8400 99-011, 340 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of palladium Pd, lead Pb, tin Sn, antimony Sb, niobium Nb and bismuth Bi in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system of high-level radioactive wastes. Besides treating hydrolysis in detail, this report focuses on the formation of complexes or compounds with chloride, fluoride, carbonate, nitrate, sulfate and phosphate. Other important inorganic ligands (sulfide for lead and antimony, ammonia for palladium) are also included. In this study, the specific ion interaction theory (SIT) approach is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Rai, D.*; Rao, L.*; Weger, H. T.*; Felmy, A. R.*; Choppin, G. R.*; Yui, Mikazu
JNC TN8400 99-009, 115 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Th(IV), U(IV), Np(IV), and Pu(IV) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(IV) species was lacking, the data were selected based on chemical analogy to other tetravalent actinides. ln this study, the Pitzer ion-interaction model is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.