Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 40(2), p.347 - 352, 2024/02
Times Cited Count:2 Percentile:23.37(Chemistry, Analytical)The Eu(III) distribution mechanism in single extractant-impregnated polymer-layered silica particle in a complex solution containing multiple lanthanide ions was investigated using fluorescence microspectroscopy. The rate-determining step was the reaction of Eu(III) with the two extractant molecules. The obtained mechanism and rate constants agreed with those of the single-ion distribution system, in which Eu(III) was distributed to the particles in the Eu(III) solution.
 -tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degree
-tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degreeMiyagawa, Akihisa*; Takahashi, Takumi*; Kuzure, Yoshiaki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 39(11), p.1929 - 1936, 2023/11
Times Cited Count:1 Percentile:10.71(Chemistry, Analytical)In this study, we revealed the Eu(III) distribution in a single diglycolamide-derivative extractant (TODGA)-impregnated polymer-coated silica particle. The reaction of Eu(III) with two TODGA molecules in the polymer layer was the rate-limiting process, as evidenced by the absence of any correlation between the rate constants (k and k
and k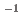 ) and concentrations of Eu(III) and HNO
) and concentrations of Eu(III) and HNO .
.
Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 96(9), p.1019 - 1025, 2023/09
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)In the present study, we have elucidated the mass transfer mechanism of Eu(III) and Sm(III) in the solution with these ions in single nitrilotriacetamide (NTA) extractant-impregnated polymer-coated silica particle. The rate-limiting process of mass transfer was the reaction process of ions with NTA molecules, in which the NO
 ions were not involved, which was consistent with that obtained in single ion distribution system.
ions were not involved, which was consistent with that obtained in single ion distribution system.
Miyagawa, Akihisa*; Hayashi, Naoki*; Kuzure, Yoshiaki*; Takahashi, Takumi*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; et al.
Bulletin of the Chemical Society of Japan, 96(7), p.671 - 676, 2023/07
Times Cited Count:8 Percentile:61.41(Chemistry, Multidisciplinary)We investigated the distribution mechanism of Eu(III) in a single polymer-coated silica particle including nitrilotriacetamide (NTA) extractants known as HONTA and TOD2EHNTA. The present study provides a valuable approach for the evaluation and enhancement of the functionality of "single extractant-impregnated polymer-coated silica particle".
Simonnet, M.; Sasaki, Yuji; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 41(7), p.857 - 867, 2023/00
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)Kirishima, Akira*; Terasaki, Mariko*; Miyakawa, Kazuya; Okamoto, Yoshihiro; Akiyama, Daisuke*
Chemosphere, 289, p.133181_1 - 133181_12, 2022/04
Times Cited Count:1 Percentile:3.45(Environmental Sciences)no abstracts in English
Matsutani, Takafumi; Sasaki, Yuji; Katsuta, Shoichi*
Analytical Sciences, 37(11), p.1603 - 1609, 2021/11
Times Cited Count:7 Percentile:37.70(Chemistry, Analytical)We investigated the chemical behavior of lanthanides (Ln) using diglycolamide extractant with multistage extraction. We obtained the breakthrough curves for light and middle Ln. Our study reveals that the metal extraction limit depends on their  values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO
values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO , organic phase: 0.1 M TDDGA (
, organic phase: 0.1 M TDDGA ( -tetradecyl-diglycolamide) in
-tetradecyl-diglycolamide) in  -dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.
-dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.
Simonnet, M.; Kobayashi, Toru; Shimojo, Kojiro; Yokoyama, Keiichi; Yaita, Tsuyoshi
Inorganic Chemistry, 60(17), p.13409 - 13418, 2021/09
Times Cited Count:25 Percentile:91.11(Chemistry, Inorganic & Nuclear)Simonnet, M.; Suzuki, Shinichi; Miyazaki, Yuji*; Kobayashi, Toru; Yokoyama, Keiichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 38(4), p.430 - 440, 2020/00
Times Cited Count:23 Percentile:68.08(Chemistry, Multidisciplinary)Naganawa, Hirochika; Suzuki, Hideya*; Noro, Junji*; Kimura, Takaumi
Chemical Communications, (23), p.2963 - 2965, 2005/06
A "superweak" anion, TFPB-, gives rise to a field effect on the selectivity for Am over Ln
over Ln in their extraction from aqueous HNO
in their extraction from aqueous HNO solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
Hayashi, Hirokazu; Minato, Kazuo
Journal of Physics and Chemistry of Solids, 66(2-4), p.422 - 426, 2005/02
Times Cited Count:34 Percentile:74.82(Chemistry, Multidisciplinary)Stability of lanthanide sesquioxides (Ln O
O ; Ln=La, Nd, Gd) in LiCl-KCl eutectic melt is studied. Some lanthanide oxides have been pointed out to form oxide chlorides (LnOCl) in the chloride melts, according to the reaction, Ln
; Ln=La, Nd, Gd) in LiCl-KCl eutectic melt is studied. Some lanthanide oxides have been pointed out to form oxide chlorides (LnOCl) in the chloride melts, according to the reaction, Ln O
O + 2Cl
+ 2Cl = 2LnOCl +O
= 2LnOCl +O . Equilibrium of the reaction for Ln = La, Nd, Gd in LiCl-KCl eutectic melt were studied. Entire La
. Equilibrium of the reaction for Ln = La, Nd, Gd in LiCl-KCl eutectic melt were studied. Entire La O
O and a part of Nd
and a part of Nd O
O converted to LaOCl and NdOCl respectively, though Gd
converted to LaOCl and NdOCl respectively, though Gd O
O remained with a trace amount of GdOCl for 2wt % of Ln
remained with a trace amount of GdOCl for 2wt % of Ln O
O in LiCl-KCl eutectic melt. The equilibrium depends on the free energies of formation of the solid compounds, Ln
in LiCl-KCl eutectic melt. The equilibrium depends on the free energies of formation of the solid compounds, Ln O
O and LnOCl, and that of oxide ion (O
and LnOCl, and that of oxide ion (O ) in the melt. We derive the chemical potential of the oxide ion from the equilibrium using the reported thermochemical data of Ln
) in the melt. We derive the chemical potential of the oxide ion from the equilibrium using the reported thermochemical data of Ln O
O and LnOCl.
and LnOCl.
Lis, S.*; Kimura, Takaumi; Yoshida, Zenko; But, S.*
Journal of Alloys and Compounds, 380(1-2), p.173 - 176, 2004/10
Times Cited Count:6 Percentile:40.24(Chemistry, Physical)no abstracts in English
Fu, F.; Akagi, Tasuku*; Suzuki, Yuichiro*; Watanabe, Kazuo; Yabuki, Sadayo*
Geochemical Journal, 38(4), p.333 - 343, 2004/08
Times Cited Count:9 Percentile:19.45(Geochemistry & Geophysics)no abstracts in English
Tian, G.*; Kimura, Takaumi; Yoshida, Zenko; Zhu, Y.*; Rao, L.*
Radiochimica Acta, 92(8), p.495 - 499, 2004/08
Times Cited Count:17 Percentile:71.02(Chemistry, Inorganic & Nuclear)no abstracts in English
 -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from nitric acid solutions
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from nitric acid solutionsShimada, Asako; Yaita, Tsuyoshi; Narita, Hirokazu; Tachimori, Shoichi; Okuno, Kenji*
Solvent Extraction and Ion Exchange, 22(2), p.147 - 161, 2004/03
Times Cited Count:68 Percentile:81.34(Chemistry, Multidisciplinary)The distribution ratios of lanthanides with  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from 1-5M nitric acid solutions were determined and the extraction mechanism was discussed on the basis of the slope analyses of acid and ligand concentration dependencies and lanthanide patterns (variation of the distribution ratio as a function of ionic radius). The lanthanide extractions were explained through two mechanisms from the standpoint of the formation of the extracted complex as follows: (1) the formation of inner-sphere complex with two DMDPhPDA molecules for light lanthanides in the extraction from HNO
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from 1-5M nitric acid solutions were determined and the extraction mechanism was discussed on the basis of the slope analyses of acid and ligand concentration dependencies and lanthanide patterns (variation of the distribution ratio as a function of ionic radius). The lanthanide extractions were explained through two mechanisms from the standpoint of the formation of the extracted complex as follows: (1) the formation of inner-sphere complex with two DMDPhPDA molecules for light lanthanides in the extraction from HNO less than 3M, and (2) the formation of outer-sphere complex with the third DMDPhPDA molecules in addition to the inner-sphere complex in the extraction of light lanthanides from HNO
less than 3M, and (2) the formation of outer-sphere complex with the third DMDPhPDA molecules in addition to the inner-sphere complex in the extraction of light lanthanides from HNO more than 3M, and those of heavy lanthanides from 1-5M HNO
more than 3M, and those of heavy lanthanides from 1-5M HNO solutions. Nitric acid concentration is more influential than the ligand concentration in the formation of outer-sphere complex.
solutions. Nitric acid concentration is more influential than the ligand concentration in the formation of outer-sphere complex.
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Uchiyama, Gunzo*
Journal of Nuclear Science and Technology, 39(Suppl.3), p.874 - 877, 2002/11
To facilitate the management of high-level liquid waste (HLLW) and minimize its long-term radiological risk in geologic disposal, we have proposed an advanced partitioning process by extraction chromatography using a minimal organic solvent and compact equipment to separate long-lived minor actinides (MA) and specific fission products (FP) such as Zr and Mo from nitrate acidic HLLW solution. Novel silica-based extraction-resin for elemental groups separation was prepared by impregnating CMPO (octyl(phenyl)-N, N-diisobutylcarbamoylmethylphosphine oxide) into a macro-reticular styrene-divinylbenzene copolymer immobilized in porous silica particles with a diameter of 50  m (SiO
m (SiO -P). Separation experiments for simulated HLLW solutions containing a trace amount of
-P). Separation experiments for simulated HLLW solutions containing a trace amount of  Am (III) and macro amounts of typical FP elements were carried out by column chromatography. It was found that the elements in the simulated HLLW were successfully separated to the following three groups: Cs-Sr-Rh-Ru, Pd-Ln-Am and Zr-Mo.
Am (III) and macro amounts of typical FP elements were carried out by column chromatography. It was found that the elements in the simulated HLLW were successfully separated to the following three groups: Cs-Sr-Rh-Ru, Pd-Ln-Am and Zr-Mo.
Narita, Hirokazu; Yaita, Tsuyoshi; Okamoto, Yoshihiro; Takai, Konomi; Shiwaku, Hideaki; Shimada, Asako*; Yamamoto, Takeshi*; Tanida, Hajime*
Spring-8 Experiment Report, Vol.6, P. 9, 2001/00
no abstracts in English
Kobayashi, Fumiaki; Ogawa, Toru; Akabori, Mitsuo;
Journal of the American Ceramic Society, 78(8), p.2279 - 2281, 1995/00
Times Cited Count:21 Percentile:67.98(Materials Science, Ceramics)no abstracts in English
Usuda, Shigekazu; Abe, Hitoshi; Tachimori, Shoichi; Takeishi, Hideyo;
Solvent Extraction 1990, p.717 - 722, 1992/00
no abstracts in English
; Kimura, Takaumi
Journal of Nuclear Science and Technology, 28(8), p.780 - 783, 1991/08
no abstracts in English