Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Saito, Shingo*; Haraga, Tomoko; Marumo, Kazuki*; Sato, Yoshiyuki; Nakano, Yuta*; Tasaki-Handa, Yuiko*; Shibukawa, Masami*
Bulletin of the Chemical Society of Japan, 96(3), p.223 - 225, 2023/03
Times Cited Count:3 Percentile:32.03(Chemistry, Multidisciplinary)Highly efficient and effective separation between americium (Am ) and curium ion (Cm
) and curium ion (Cm ) was achieved by two simple electrophoresis-based techniques. Am
) was achieved by two simple electrophoresis-based techniques. Am and Cm
and Cm ions were complexed with fluorophore-modified acyclic hexadentate and octadentate polyaminocarboxylates and then were electrophoretically separated and fluorescently detected in free solution with ternary complexation or in gel medium.
ions were complexed with fluorophore-modified acyclic hexadentate and octadentate polyaminocarboxylates and then were electrophoretically separated and fluorescently detected in free solution with ternary complexation or in gel medium.
Collaborative Laboratories for Advanced Decommissioning Science; Tokyo Institute of Technology*
JAEA-Review 2022-028, 54 Pages, 2022/11
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2021. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2019, this report summarizes the research results of the "Development of Tailor-made Adsorbents for Uranium Recovery from Seawater on the Basis of Uranyl Coordination Chemistry" conducted from FY2019 to FY2021. Since the final year of this proposal was FY2021, the results for three fiscal years were summarized. The present study aims to develop a new ligand class for efficient and selective capture of uranium from seawater. On the basis of deep understanding on uranyl coordination chemistry, we design molecular structures of pentadentate ligands as functional moieties for uranium adsorption from seawater and study fundamental coordination chemistry of uranyl ion with those ligands in order to resolve current problems in uranium recovery technology …
Collaborative Laboratories for Advanced Decommissioning Science; Tokyo Institute of Technology*
JAEA-Review 2021-041, 42 Pages, 2022/01
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2020. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2019, this report summarizes the research results of the "Development of tailor-made adsorbents for uranium recovery from seawater on the basis of uranyl coordination chemistry" conducted in FY2020. On the basis of deep understanding on uranyl coordination chemistry, we design molecular structures of pentadentate ligands as functional moieties for uranium adsorption from seawater and study coordination chemistry of uranyl ion with those ligands in order to resolve current problems in uranium recovery technology from seawater and to develop novel selective and efficient adsorbents for this purpose.
Ochs, M.*; Dolder, F.*; Tachi, Yukio
Applied Geochemistry, 136, p.105161_1 - 105161_11, 2022/01
Times Cited Count:9 Percentile:67.78(Geochemistry & Geophysics)Various types of radioactive wastes and environments contain organic substances that can stabilize the aqueous complexes with radionuclides and therefore lead to a decrease of sorption. The present study focuses on testing a methodology to quantify sorption reduction factors (SRFs) in the presence of organic ligands for cement systems. Three approaches for the estimation of SRFs; (1) analogy with solubility enhancement factors, (2) radionuclide speciation based on the thermodynamic calculations, and (3) experimental sorption data in ternary systems, were coupled and tested for the representative organic ligands (ISA and EDTA) and selected key radionuclides (actinides). Our approach allows to critically evaluate the dependence of SRFs for various systems on the chosen method of quantification, in accordance with the data availability for a given systems. The reliable SRFs can only be derived from the sorption measurements in ternary systems. SRF often need to be derived in the absence of such direct evidence, and estimations need to be made based on analogies and speciation information. However, such estimates may be subject to substantial uncertainties.
Collaborative Laboratories for Advanced Decommissioning Science; Tokyo Institute of Technology*
JAEA-Review 2020-026, 41 Pages, 2020/12
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2019. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2019, this report summarizes the research results of the "Development of Tailor-Made Adsorbents for Uranium Recovery from Seawater on the Basis of Uranyl Coordination Chemistry". On the basis of deep understanding on uranyl coordination chemistry, we design molecular structures of pentadentate ligands as functional moieties for uranium adsorption from seawater and study coordination chemistry of uranyl ion with those ligands in order to resolve current problems in uranium recovery technology from seawater and to develop novel selective and efficient adsorbents for this purpose.
Tachi, Yukio; Ochs, M.*
Progress in Nuclear Science and Technology (Internet), 5, p.229 - 232, 2018/11
Various types of post-accident radioactive waste have been generated from cleanup and decommissioning activities at the Fukushima Daiichi Nuclear Power Plant. For the disposal of these wastes, perturbation effects resulting from co-existing substances (e.g., organic substances, boron, and salts) are needed to be considered. Such co-existing substances may influence on the radionuclide sorption parameters for the safety assessment of the disposal systems. The present study focuses on developing the methodology to quantify sorption parameters by considering such perturbation effects and illustrating example calculations regarding the sorption reduction factors (SRFs) due to the presence of organic ligands (ISA) for cement systems. Three approaches for the derivations of SRFs for cement-Am-ISA case were compared. These options should be applied as a stepwise manner according to the data availability for the perturbation effects resulting from the co-existing substances.
Ochs, M.*; Vriens, B.*; Tachi, Yukio
Progress in Nuclear Science and Technology (Internet), 5, p.208 - 212, 2018/11
The clean-up activities related to the accident at the Fukushima Nuclear Power Plant give rise to several types of wastes containing cementitious materials, such as concrete. Further, the use of cement-based barriers may be considered, due to their favorable and stable chemical properties, including their ability to sorb or incorporate radionuclides. Wastes from Fukushima are expected to contain substances that can have perturbing effects on retention, especially organic complexing substances, boron, and chloride salts. The present study focuses on a methodology for quantifying the retention behaviour of UVI) and U(IV) in cement materials of different degradation and in the presence of organics, boron, and salts on the basis of available literature information. A stepwise approach is proposed and illustrated for Kd setting for U(VI) and U(IV).
Awual, M. R.; Yaita, Tsuyoshi; Miyazaki, Yuji; Matsumura, Daiju; Shiwaku, Hideaki; Taguchi, Tomitsugu
Scientific Reports (Internet), 6, p.19937_1 - 19937_10, 2016/01
Times Cited Count:201 Percentile:97.15(Multidisciplinary Sciences)Awual, M. R.; Eldesoky, G. E.*; Yaita, Tsuyoshi; Naushad, M.*; Shiwaku, Hideaki; Alothman, Z. A.*; Suzuki, Shinichi
Chemical Engineering Journal, 279, p.639 - 647, 2015/11
Times Cited Count:260 Percentile:99.16(Engineering, Environmental) -dimethylformamide
-dimethylformamideKim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Ikeda, Yasuhisa*
Journal of Alloys and Compounds, 408-412, p.1291 - 1295, 2006/02
Times Cited Count:10 Percentile:53.71(Chemistry, Physical)The electrochemical reactions of UO (
( -diketonato)
-diketonato) DMF, UO
DMF, UO (trop)
(trop) DMF and UO
DMF and UO (sap)(DMF)
(sap)(DMF) , (DMF = N,N-dimethylformamide,
, (DMF = N,N-dimethylformamide,  -diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E
-diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E ,
, 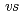 . ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO
. ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO (ttfa)
(ttfa) DMF, -1.18 V for UO
DMF, -1.18 V for UO (btfa)
(btfa) DMF, -1.46 V for UO
DMF, -1.46 V for UO (dbm)
(dbm) DMF, -1.46 V for UO
DMF, -1.46 V for UO (trop)
(trop) DMF, and -1.59 V for UO
DMF, and -1.59 V for UO (sap)(DMF)
(sap)(DMF) .
.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Uchiyama, Gunzo*; Ikeda, Yasuhisa*
Radiochimica Acta, 93(2), p.75 - 81, 2005/02
Times Cited Count:9 Percentile:51.72(Chemistry, Inorganic & Nuclear)no abstracts in English
Krot, N. N.*; Saeki, Masakatsu
JAERI-Review 2003-005, 37 Pages, 2003/03
The original manuscript was prepared by Professor N. N. Krot of Institute of Physical Chemistry, Russian Academy of sciences, in 1997. Saeki tried to translate that into Japanese and to add some new data since 1997. The contents include the whole picture of cation-cation interactions mainly on 5-valence neptunium compounds. Firstly, characteristic structures are summarized for the cation-cation bonding in compounds of neptunium. Secondly, it is introduced how the cation-cation bonding affects physical and chemical properties of the compounds. Then, detection-methods are shown for the cation-cation bonding in the compounds. Besides, the cation-cation interaction are shortly reviewed for compounds of other actinide-ions.
Wei, Y.*; Hoshi, Harutaka*; Kumagai, Mikio*; Asakura, Toshihide; Uchiyama, Gunzo*
Journal of Nuclear Science and Technology, 39(Suppl.3), p.761 - 764, 2002/11
To separate long-lived minor actinides and specific fission products such as Zr and Mo from nitrate acidic high-level liquid waste, we studied an advanced partitioning process by extraction chromatography using minimal organic solvent and compact equipment. In this work, we synthesized several new type of nitrogen donor ligands, 2,6-bi-(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) with different alkyl groups and prepared novel silica-based extraction-resins by impregnating these ligands into the SiO -P support with a diameter of 50
-P support with a diameter of 50  m. The adsorption performance of
m. The adsorption performance of  Am and Ln (III) from nitrate solution was investigated. It was found that the adsorption behavior depends strongly on the alkyl group in R-BTP.
Am and Ln (III) from nitrate solution was investigated. It was found that the adsorption behavior depends strongly on the alkyl group in R-BTP.  Bu-BTP/SiO
Bu-BTP/SiO -P and
-P and  Hex-BTP/SiO
Hex-BTP/SiO -P showed high absorbability and selectivity for Am (III) over Ln (III). The separation factor is about 10
-P showed high absorbability and selectivity for Am (III) over Ln (III). The separation factor is about 10 for Am/Ce and near 10
for Am/Ce and near 10 for Am/Eu-Gd, respectively. Effective Am (III) separation form Ln (III) by extraction chromatography using R-BTP/SiO
for Am/Eu-Gd, respectively. Effective Am (III) separation form Ln (III) by extraction chromatography using R-BTP/SiO -P extraction-resins is expected.
-P extraction-resins is expected.
; Saito, Keiichiro; Shiba, Koreyuki
Journal of Nuclear Science and Technology, 20(5), p.439 - 440, 1983/00
Times Cited Count:56 Percentile:99.14(Nuclear Science & Technology)no abstracts in English
Ono, Shinichi
Kagaku No Ryoiki, 27(7), p.562 - 567, 1973/07
no abstracts in English
Ochs, M.*; Dolder, F.*; Tachi, Yukio
no journal, ,