Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ebihara, Kenichi; Fujihara, Hiro*; Shimizu, Kazuyuki*; Yamaguchi, Masatake; Toda, Hiroyuki*
International Journal of Hydrogen Energy, 136, p.751 - 756, 2025/06
Times Cited Count:0It has been experimentally reported that adding tin (Sn) to high-strength aluminum-zinc-magnesium (Al-Zn-Mg) alloys effectively suppresses hydrogen (H) embrittlement, which may be attributed to H absorption by the second-phase particles of Sn. To verify this fact, a simulation of H entry into the Sn phase in Al was performed using a model based on the reaction-diffusion equation that incorporates the solid solution energy of H evaluated by first-principles calculations. The results showed that the H solid solution site concentration of the second-phase particles must be at least five times higher than that of the Al phase for H absorption by the Sn second-phase particles to suppress H embrittlement. Therefore, the actual H embrittlement suppression effect of Sn second-phase particles is limited, and other factors may influence the suppression of H embrittlement in the experiment.
Higa, Ryota*; Fujihara, Hiro*; Toda, Hiroyuki*; Kobayashi, Masakazu*; Ebihara, Kenichi; Takeuchi, Akihisa*
Materials Transactions, 65(8), p.899 - 906, 2024/08
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)It is indispensable to suppress hydrogen embrittlement (HE) to develop the strength of the Al-Zn-Mg alloy. Because intergranular fracture (IGF) is mainly observed when HE occurs in the alloy, we need to understand the IGF initiation to suppress HE. In the present study, we investigated the stress, strain, and H concentration, which influence the IGF initiation, in actual fractured regions by simulation of a crystal plasticity finite element method and H diffusion analysis in a 3D image-based model, which was created based on 3D polycrystalline microstructure data obtained from X-ray imaging technique. Combining the simulation and in-situ observation of the tensile test sample by X-ray CT, we examined the stress, strain, and H concentration, and discussed the IG crack initiation condition. As a result, it is revealed that stress normal to grain boundary induced by crystal plasticity dominates IG crack initiation while the accumulation of H due to stress has little impact on it.
 Mg,
Mg,  Si,
Si,  Fe,
Fe,  Cu, and
Cu, and  Zn
ZnSugihara, Kenta*; Meigo, Shinichiro; Iwamoto, Hiroki; Maekawa, Fujio
Nuclear Instruments and Methods in Physics Research B, 549, p.165299_1 - 165299_12, 2024/04
Times Cited Count:0 Percentile:0.00(Instruments & Instrumentation)Fujihara, Hiro*; Toda, Hiroyuki*; Ebihara, Kenichi; Kobayashi, Masakazu*; Mayama, Tsuyoshi*; Hirayama, Kyosuke*; Shimizu, Kazuyuki*; Takeuchi, Akihisa*; Uesugi, Masayuki*
International Journal of Plasticity, 174, p.103897_1 - 103897_22, 2024/03
Times Cited Count:9 Percentile:93.27(Engineering, Mechanical)Hydrogen(H) embrittlement in high-strength aluminum(Al) alloys is a crucial problem. H accumulation at the interface of precipitates in Al alloy is considered to cause embrittlement. However, there is no quantitative knowledge regarding the interaction between H distribution and stress field near cracks. In this study, using a multi-modal three-dimensional image-based simulation combining the crystal plasticity finite element method and H diffusion analysis, we tried to capture the stress distribution near the crack, its influence on the H distribution, and the probability of crack initiation in the experimental condition. As a result, it was found that grain boundary cracks transition to quasi-cleavage cracks in the region where the cohesive energy of the semi-coherent interface of MgZn precipitates decreases due to H accumulation near the tip. We believe the present simulation method successfully bridges nanoscale delamination and macroscale brittle fracture.
precipitates decreases due to H accumulation near the tip. We believe the present simulation method successfully bridges nanoscale delamination and macroscale brittle fracture.
Hagihara, Koji*; Mayama, Tsuyoshi*; Yamasaki, Michiaki*; Harjo, S.; Tokunaga, Toko*; Yamamoto, Kazuki*; Sugita, Mika*; Aoyama, Kairi*; Gong, W.; Nishimoto, Soya*
International Journal of Plasticity, 173, p.103865_1 - 103865_21, 2024/02
Times Cited Count:24 Percentile:98.62(Engineering, Mechanical) condensed state in
condensed state in  Mg using the
Mg using the  C+
C+ C scattering
C scatteringFujikawa, Y.*; Kawabata, T.*; Adachi, S.*; Hirose, Kentaro; Makii, Hiroyuki; Nishio, Katsuhisa; Orlandi, R.; Suzaki, Fumi; 13 of others*
Physics Letters B, 848, p.138384_1 - 138384_6, 2024/01
Times Cited Count:6 Percentile:73.96(Astronomy & Astrophysics)Tang, J.*; Wang, Y.*; Fujihara, Hiro*; Shimizu, Kazuyuki*; Hirayama, Kyosuke*; Ebihara, Kenichi; Takeuchi, Akihisa*; Uesugi, Masayuki*; Toda, Hiroyuki*
Scripta Materialia, 239, p.115804_1 - 115804_5, 2024/01
Times Cited Count:11 Percentile:80.41(Nanoscience & Nanotechnology)Stress corrosion cracking (SCC) behaviors induced by the combination of external and internal hydrogen (H) in an Al-Zn-Mg-Cu alloy were systematically investigated via in situ 3D characterization techniques. SCC of the Al-Zn-Mg-Cu alloy could initiate and propagate in the potential crack region where the H concentration exceeded a critical value, in which the nanoscopic H-induced decohesion of 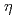 -MgZn
-MgZn precipitates resulted in macroscopic cracking. External H that penetrated the alloy from the environment played a crucial role during the SCC of the Al-Zn-Mg-Cu alloy by generating gradient-distributed H-affected zones near the crack tips, which made Al alloys in water environment more sensitive to SCC. Additionally, the pre-existing internal H was driven toward the crack tips during plastic deformation. It was involved in the SCC and made contributions to both the cracks initiation and propagation.
precipitates resulted in macroscopic cracking. External H that penetrated the alloy from the environment played a crucial role during the SCC of the Al-Zn-Mg-Cu alloy by generating gradient-distributed H-affected zones near the crack tips, which made Al alloys in water environment more sensitive to SCC. Additionally, the pre-existing internal H was driven toward the crack tips during plastic deformation. It was involved in the SCC and made contributions to both the cracks initiation and propagation.
Higa, Ryota*; Fujihara, Hiro*; Toda, Hiroyuki*; Kobayashi, Masakazu*; Ebihara, Kenichi; Takeuchi, Akihisa*
Keikinzoku, 73(11), p.530 - 536, 2023/11
In Al-Zn-Mg alloys, suppression of hydrogen embrittlement is necessary to improve their strength. In this study, the distribution of stress, strain, and hydrogen concentration in the actual fracture region was investigated using the crystal plasticity finite element method and hydrogen diffusion analysis based on a model derived from three-dimensional polycrystalline microstructural data obtained by X-ray CT. In addition, the distributions of stress, strain, and hydrogen concentration were compared with the actual crack initiation behavior by combining in-situ observation of tensile tests using X-ray CT and simulation. The results show that stress loading perpendicular to the grain boundary due to crystal plasticity dominates grain boundary crack initiation. It was also found that internal hydrogen accumulation due to crystal plasticity has little effect on crack initiation.
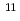 B-enriched MgB
B-enriched MgB wire using a pulsed neutron source
wire using a pulsed neutron sourceMachiya, Shutaro*; Osamura, Kozo*; Hishinuma, Yoshimitsu*; Taniguchi, Hiroyasu*; Harjo, S.; Kawasaki, Takuro
Quantum Beam Science (Internet), 7(4), p.34_1 - 34_17, 2023/10
 frameworks
frameworksShimokawa, Kohei*; Hatakeyama, Takuya*; Li, H.*; Ichitsubo, Tetsu
Current Opinion in Electrochemistry, 38, p.101209_1 - 101209_8, 2023/04
Times Cited Count:6 Percentile:22.85(Chemistry, Physical)Ye, X.*; Shimokawa, Kohei*; Kezuka, Yuto*; Hatakeyama, Takuya*; Li, H.*; Ichitsubo, Tetsu
Journal of Physical Chemistry C, 127(11), p.5210 - 5218, 2023/03
Times Cited Count:2 Percentile:20.33(Chemistry, Physical) @Mg(OH)
@Mg(OH) ) into porous media for Cr(VI) removal from groundwater
) into porous media for Cr(VI) removal from groundwaterMaamoun, I.; Falyouna, O.*; Eljamal, R.*; Idham, M. F.*; Tanaka, Kazuya; Eljamal, O.*
Chemical Engineering Journal, 451, Part3, p.138718_1 - 138718_22, 2023/01
Times Cited Count:46 Percentile:92.38(Engineering, Environmental) -ray emitter
-ray emitter 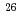 Al in massive stars; Study of the key
Al in massive stars; Study of the key 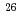 Al(n, p) reaction
Al(n, p) reactionLederer-Woods, C.*; Harada, Hideo; Kimura, Atsushi; 121 of others*
Physical Review C, 104(2), p.L022803_1 - L022803_7, 2021/08
Times Cited Count:8 Percentile:67.39(Physics, Nuclear)Zhang, X. X.*; Andr , H.*; Harjo, S.; Gong, W.*; Kawasaki, Takuro; Lutz, A.*; Lahres, M.*
, H.*; Harjo, S.; Gong, W.*; Kawasaki, Takuro; Lutz, A.*; Lahres, M.*
Materials & Design, 198, p.109339_1 - 109339_9, 2021/01
Times Cited Count:58 Percentile:93.33(Materials Science, Multidisciplinary) Si single crystals
Si single crystalsHayashi, Kei*; Saito, Wataru*; Sugimoto, Kazuya*; Oyama, Kenji*; Hayashi, Koichi*; Happo, Naohisa*; Harada, Masahide; Oikawa, Kenichi; Inamura, Yasuhiro; Miyazaki, Yuzuru*
AIP Advances (Internet), 10(3), p.035115_1 - 035115_7, 2020/03
Times Cited Count:19 Percentile:68.77(Nanoscience & Nanotechnology)Hosokawa, Shinya*; Kimura, Koji*; Yamasaki, Michiaki*; Kawamura, Yoshihito*; Yoshida, Koji*; Inui, Masanori*; Tsutsui, Satoshi*; Baron, A. Q. R.*; Kawakita, Yukinobu; Ito, Shinichi*
Journal of Alloys and Compounds, 695, p.426 - 432, 2017/02
Times Cited Count:5 Percentile:22.82(Chemistry, Physical) = 21 between normal and intruder configurations
= 21 between normal and intruder configurationsLic , R.*; Rotaru, F.*; Borge, M. J. G.*; Gr
, R.*; Rotaru, F.*; Borge, M. J. G.*; Gr vy, S.*; Negoita, F.*; Poves, A.*; Sorlin, O.*; Andreyev, A. N.; Borcea, R.*; Costache, C.*; et al.
vy, S.*; Negoita, F.*; Poves, A.*; Sorlin, O.*; Andreyev, A. N.; Borcea, R.*; Costache, C.*; et al.
Physical Review C, 95(2), p.021301_1 - 021301_6, 2017/02
Times Cited Count:19 Percentile:79.02(Physics, Nuclear) superconductor
superconductorMachida, Masahiko; Koyama, Tomio*; Kato, Masaru*; Ishida, Takekazu*
Nuclear Instruments and Methods in Physics Research A, 559(2), p.594 - 596, 2006/04
Times Cited Count:11 Percentile:58.92(Instruments & Instrumentation)no abstracts in English
Hayashi, Takao; Tobita, Kenji; Nishio, Satoshi; Ikeda, Kazuki*; Nakamori, Yuko*; Orimo, Shinichi*; DEMO Plant Design Team
Fusion Engineering and Design, 81(8-14), p.1285 - 1290, 2006/02
Times Cited Count:27 Percentile:84.58(Nuclear Science & Technology)Neutron transport calculations were carried out to evaluate the capability of metal hydrides and borohydrides as an advanced shielding material. Some hydrides indicated considerably higher hydrogen content than polyethylene and solid hydrogen. The hydrogen-rich hydrides show superior neutron shielding capability to the conventional materials. From the temperature dependence of dissociation pressure, ZrH and TiH
and TiH can be used without releasing hydrogen at the temperature of less than 640
can be used without releasing hydrogen at the temperature of less than 640  C at 1 atm. ZrH
C at 1 atm. ZrH and Mg(BH
and Mg(BH )
) can reduce the thickness of the shield by 30% and 20% compared to a combination of steel and water, respectively. Mixing some hydrides with F82H produces considerable effects in
can reduce the thickness of the shield by 30% and 20% compared to a combination of steel and water, respectively. Mixing some hydrides with F82H produces considerable effects in  -ray shielding. The neutron and
-ray shielding. The neutron and  -ray shielding capabilities decrease in order of ZrH
-ray shielding capabilities decrease in order of ZrH
 Mg(BH
Mg(BH )
) and F82H
and F82H  TiH
TiH and F82H
and F82H  water and F82H.
water and F82H.

Machida, Masahiko; Koyama, Tomio*; Kato, Masaru*; Ishida, Takekazu*
Physica C, 426-431(1), p.169 - 173, 2005/10
no abstracts in English