Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakamura, Shoji; Shibahara, Yuji*; Endo, Shunsuke; Kimura, Atsushi
Journal of Nuclear Science and Technology, 59(11), p.1388 - 1398, 2022/11
Times Cited Count:1 Percentile:11.89(Nuclear Science & Technology)The present study selected  Np among radioactive nuclides and aimed to measure the thermal-neutron capture cross-section for
Np among radioactive nuclides and aimed to measure the thermal-neutron capture cross-section for  Np in a well-thermalized neutron field by an activation method. A
Np in a well-thermalized neutron field by an activation method. A  Np standard solution was used for irradiation samples. A thermal-neutron flux at an irradiation position was measured with neutron flux monitors:
Np standard solution was used for irradiation samples. A thermal-neutron flux at an irradiation position was measured with neutron flux monitors: 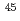 Sc,
Sc, 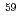 Co,
Co, 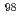 Mo,
Mo,  Ta and
Ta and 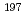 Au. The
Au. The  Np sample and flux monitors were irradiated together for 30 minutes in the graphite thermal column equipped with the Kyoto University Research Reactor. The similar irradiation was carried out twice. After the irradiations, the
Np sample and flux monitors were irradiated together for 30 minutes in the graphite thermal column equipped with the Kyoto University Research Reactor. The similar irradiation was carried out twice. After the irradiations, the  Np samples were quantified using 312-keV gamma ray emitted from
Np samples were quantified using 312-keV gamma ray emitted from  Pa in a radiation equilibrium with
Pa in a radiation equilibrium with  Np. The reaction rates of
Np. The reaction rates of  Np were obtained from gamma-ray peak net counts given by
Np were obtained from gamma-ray peak net counts given by  Np, and then the thermal-neutron capture cross-section of
Np, and then the thermal-neutron capture cross-section of  Np was found to be 173.8
Np was found to be 173.8 4.4 barn by averaging the results obtained by the two irradiations. The present result was in agreement with the reported data given by a time-of-flight method within the limit of uncertainty.
4.4 barn by averaging the results obtained by the two irradiations. The present result was in agreement with the reported data given by a time-of-flight method within the limit of uncertainty.
 Np(n,
Np(n,  ) reaction with TC-Pn in KUR
) reaction with TC-Pn in KURNakamura, Shoji; Endo, Shunsuke; Kimura, Atsushi; Shibahara, Yuji*
KURNS Progress Report 2021, P. 93, 2022/07
In terms of nuclear transmutation studies of minor actinides in nuclear wastes, the present work selected  Np among them and aimed to measure the thermal-neutron capture cross-section of
Np among them and aimed to measure the thermal-neutron capture cross-section of  Np using a well-thermalized neutron field by a neutron activation method because there have been discrepancies among reported cross-section data. A
Np using a well-thermalized neutron field by a neutron activation method because there have been discrepancies among reported cross-section data. A  Np standard solution was used for irradiation samples. The thermal-neutron flux at an irradiation position was measured with flux monitors:
Np standard solution was used for irradiation samples. The thermal-neutron flux at an irradiation position was measured with flux monitors: 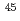 Sc,
Sc, 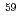 Co,
Co, 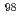 Mo,
Mo,  Ta and
Ta and 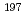 Au. The
Au. The  Np sample was irradiated together with the flux monitors for 30 minutes in the graphite thermal column equipped in the Kyoto University Research Reactor. The similar irradiation was repeated once more to confirm the reproducibility of the results. After irradiation, the
Np sample was irradiated together with the flux monitors for 30 minutes in the graphite thermal column equipped in the Kyoto University Research Reactor. The similar irradiation was repeated once more to confirm the reproducibility of the results. After irradiation, the  Np samples were quantified using 312-keV gamma-ray emitted from
Np samples were quantified using 312-keV gamma-ray emitted from  Pa in radiation equilibrium with
Pa in radiation equilibrium with  Np. The reaction rates of
Np. The reaction rates of  Np were obtained from the peak net counts of gamma-rays emitted from generated
Np were obtained from the peak net counts of gamma-rays emitted from generated  Np, and then the thermal-neutron capture cross-section of
Np, and then the thermal-neutron capture cross-section of  Np was found to be 173.8
Np was found to be 173.8 4.7 barn by averaging the results obtained by the two irradiations. The present result was in agreement with the reported data given by a time-of-flight method within a limit of uncertainty.
4.7 barn by averaging the results obtained by the two irradiations. The present result was in agreement with the reported data given by a time-of-flight method within a limit of uncertainty.
Hain, K.*; Martschini, M.*; G lce, F.*; Honda, Maki; Lachner, J.*; Kern, M.*; Pitters, J.*; Quinto, F.*; Sakaguchi, Aya*; Steier, P.*; et al.
lce, F.*; Honda, Maki; Lachner, J.*; Kern, M.*; Pitters, J.*; Quinto, F.*; Sakaguchi, Aya*; Steier, P.*; et al.
Frontiers in Marine Science (Internet), 9, p.837515_1 - 837515_17, 2022/03
Times Cited Count:12 Percentile:78.24(Environmental Sciences)Recent major advances in accelerator mass spectrometry (AMS) at the Vienna Environmental Research Accelerator (VERA) regarding detection efficiency and isobar suppression have opened possibilities for the analysis of additional long-lived radionuclides at ultra-low environmental concentrations. These radionuclides, including  U,
U,  Cs,
Cs,  Tc and
Tc and  Sr, will become important for oceanographic tracer application due to their generally conservative behavior in ocean water. In particular, the isotope ratios
Sr, will become important for oceanographic tracer application due to their generally conservative behavior in ocean water. In particular, the isotope ratios  U/
U/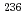 U and
U and  Cs/
Cs/ Cs have proven to be powerful fingerprints for emission source identification as they are not affected by elemental fractionation. Improved detection efficiencies allowed us to analyze all major long-lived actinides, i.e.
Cs have proven to be powerful fingerprints for emission source identification as they are not affected by elemental fractionation. Improved detection efficiencies allowed us to analyze all major long-lived actinides, i.e. 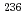 U,
U,  Np,
Np, 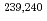 Pu,
Pu,  Am as well as the very rare
Am as well as the very rare  U, in the same 10 L water samples of an exemplary depth profile from the northwest Pacific Ocean. Especially for
U, in the same 10 L water samples of an exemplary depth profile from the northwest Pacific Ocean. Especially for  Sr analysis, our new approach has already been validated for selected reference materials (e.g. IAEA-A-12) and is ready for application in oceanographic studies. We estimate that a sample volume of only (1-3) L ocean water is sufficient for
Sr analysis, our new approach has already been validated for selected reference materials (e.g. IAEA-A-12) and is ready for application in oceanographic studies. We estimate that a sample volume of only (1-3) L ocean water is sufficient for  Sr as well as
Sr as well as  Cs analysis, respectively.
Cs analysis, respectively.
 Np
NpIrisawa, Eriko; Kato, Chiaki; Yamashita, Naoki; Sano, Naruto
Zairyo To Kankyo, 71(3), p.70 - 74, 2022/03
In order to evaluate the corrosion of stainless steels used in spent nuclear fuel reprocessing facilities, the immersion corrosion tests and polarization measurements were performed using R-SUS304ULC stainless steel in nitric acid solution containing a kind of radionuclides,  Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of
Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of 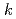 =0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
=0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
 Np
NpRovira Leveroni, G.; Katabuchi, Tatsuya*; Tosaka, Kenichi*; Matsuura, Shota*; Kodama, Yu*; Nakano, Hideto*; Iwamoto, Osamu; Kimura, Atsushi; Nakamura, Shoji; Iwamoto, Nobuyuki
Journal of Nuclear Science and Technology, 59(1), p.110 - 122, 2022/01
Times Cited Count:5 Percentile:44.62(Nuclear Science & Technology) and (U,Pu,Am,Np)O
and (U,Pu,Am,Np)O
Hirooka, Shun; Matsumoto, Taku; Kato, Masato; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*
Journal of Nuclear Materials, 542, p.152424_1 - 152424_9, 2020/12
Times Cited Count:12 Percentile:72.09(Materials Science, Multidisciplinary)The measurement of oxygen potential was conducted at 1,673, 1,773, and 1,873 K for (U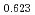 Pu
Pu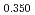 Am
Am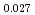 )O
)O and at 1,873 and 1,923 K for (U
and at 1,873 and 1,923 K for (U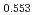 Pu
Pu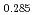 Am
Am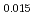 Np
Np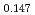 )O
)O by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO
by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
Nakamura, Shoji; Kitatani, Fumito; Kimura, Atsushi; Uehara, Akihiro*; Fujii, Toshiyuki*
Journal of Nuclear Science and Technology, 56(6), p.493 - 502, 2019/06
Times Cited Count:5 Percentile:38.60(Nuclear Science & Technology)The thermal-neutron capture cross-section(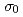 )and resonance integral(I
)and resonance integral(I ) were measured for the
) were measured for the  Np(n,
Np(n, )
) Np reaction by an activation method. A method with a Gadolinium filter, which is similar to the Cadmium difference method, was used to measure the
Np reaction by an activation method. A method with a Gadolinium filter, which is similar to the Cadmium difference method, was used to measure the 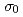 with paying attention to the first resonance at 0.489 eV of
with paying attention to the first resonance at 0.489 eV of  Np, and a value of 0.133 eV was taken as a cut-off energy. Neptunium-237 samples were irradiated at the pneumatic tube of the Kyoto University Research Reactor in Institute for Integral Radiation and Nuclear Science, Kyoto University. Wires of Co/Al and Au/Al alloys were used as monitors to determine thermal-neutron fluxes and epi-thermal Westcott's indices at an irradiation position. A
Np, and a value of 0.133 eV was taken as a cut-off energy. Neptunium-237 samples were irradiated at the pneumatic tube of the Kyoto University Research Reactor in Institute for Integral Radiation and Nuclear Science, Kyoto University. Wires of Co/Al and Au/Al alloys were used as monitors to determine thermal-neutron fluxes and epi-thermal Westcott's indices at an irradiation position. A  -ray spectroscopy was used to measure activities of
-ray spectroscopy was used to measure activities of  Np,
Np,  Np and neutron monitors. On the basis of Westcott's convention, the
Np and neutron monitors. On the basis of Westcott's convention, the 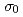 and I
and I values were derived as 186.9
values were derived as 186.9 6.2 barn, and 1009
6.2 barn, and 1009 90 barn, respectively.
90 barn, respectively.
Irisawa, Eriko; Yamamoto, Masahiro; Kato, Chiaki; Motooka, Takafumi; Ban, Yasutoshi
Journal of Nuclear Science and Technology, 56(4), p.337 - 344, 2019/04
Times Cited Count:8 Percentile:56.22(Nuclear Science & Technology)Onuki, Toshihiko; Kozai, Naofumi; Sakamoto, Fuminori; Utsunomiya, Satoshi*; Kato, Kenji*
Chemistry Letters, 46(5), p.771 - 774, 2017/05
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)The sorption behavior of Np(V) by the microbe consortia and by a single pure culture of Fe reducing bacterium was studied at pH between 3 and 7 in resting cell conditions. The sorption of Np(V) by the Fe reducing bacterium obtained in the inert condition and by the consortia in aerated condition were higher than by the Fe reducing bacterium in aerobic condition at pH below 5, strongly suggesting presence of other mechanism than the adsorption on microbial cell surface, i.e. reduction to Np(IV).
Matsueda, Makoto; Irisawa, Eriko; Kato, Chiaki; Matsui, Hiroki
Proceedings of 54th Annual Meeting of Hot Laboratories and Remote Handling (HOTLAB 2017) (Internet), 4 Pages, 2017/00
In the PUREX method, spent fuels are dissolved with nitric acid media. The reprocessing solution containing Fission Products derived from spent fuels is very corrosive to metal materials, the corrosion problem often appears on the surface stainless steel devices. The oxidizing metal ions such as Ruthenium (Ru) and Neptunium (Np) in the process solution is the key reason for severe corrosion of stainless steel. In order to obtain the corrosion rate of stainless steel, we installed the corrosion test apparatus inside an airtight concrete cell in a hot laboratory (the WAste Safety TEsting Facility (WASTEF) of the Japan Atomic Energy Agency), and performed the corrosion tests of stainless steel in the heated nitric acid solution containing Np. The corrosion tests were performed in the temperature range from room temperature to boiling point for 500 hours per batch. The results show that the presence of Np accelerate the stainless steel corrosion in the nitric acid solution.
Nakamura, Shoji; Terada, Kazushi; Shibahara, Yuji*; Uehara, Akihiro*; Fujii, Toshiyuki*; Sano, Tadafumi*; Takahashi, Yoshiyuki*; Hori, Junichi*
KURRI Progress Report 2015, P. 67, 2016/08
The activation measurements of Np-237 were performed with neutron sources at KURRI-Linac. It was found that activation measurements supported the evaluated cross-section data of JENDL-4.0.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji
Radiochimica Acta, 93(12), p.767 - 770, 2005/12
Times Cited Count:10 Percentile:55.21(Chemistry, Inorganic & Nuclear)Electrochemical and spectroelectrochemical has been used to investigate the behaviour of Neptunium (VI) in concentration 1-8 M HNO solutions. The electrochemical reactions of Np(VI) ions were found to occur quasi-reversibly. The formal redox potentials (E
solutions. The electrochemical reactions of Np(VI) ions were found to occur quasi-reversibly. The formal redox potentials (E ) for Np(VI)/Np(V) couples were determined to be +0.906, +0.908, +0.909, +0.902, +0.896, +0.895, +0.888, and +0.884 V (vs. Ag/AgCl) for Np(VI) ions in 1, 2, 3, 4, 5, 6, 7, 8 M HNO
) for Np(VI)/Np(V) couples were determined to be +0.906, +0.908, +0.909, +0.902, +0.896, +0.895, +0.888, and +0.884 V (vs. Ag/AgCl) for Np(VI) ions in 1, 2, 3, 4, 5, 6, 7, 8 M HNO solutions, respectively. The reduction processes of Np(VI) ions were followed spectroelectrochemically by using an optical transparent thin layer electrode cell. It was found that the absorption spectra measured at the applied potentials from +1.10 to +0.60 V versus Ag/AgCl reference electrode redox couple for Np(VI) in HNO
solutions, respectively. The reduction processes of Np(VI) ions were followed spectroelectrochemically by using an optical transparent thin layer electrode cell. It was found that the absorption spectra measured at the applied potentials from +1.10 to +0.60 V versus Ag/AgCl reference electrode redox couple for Np(VI) in HNO solution have clear isosbestic points. These results indicate that the reduction product of Np(VI) is Np(V), which is considerably stable in HNO
solution have clear isosbestic points. These results indicate that the reduction product of Np(VI) is Np(V), which is considerably stable in HNO solution.
solution.
Ban, Yasutoshi; Asakura, Toshihide; Morita, Yasuji
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 5 Pages, 2005/10
An idea for controlling Np behavior in the Purex process is that Np(VI) extracted by TBP is selectively reduced to Np(V) by salt-free reagents and separated from U and Pu. Allylhydrazine is expected as a selective Np(VI) reductant from a view point of reduction rates for Np(VI) and Pu(IV). To confirm the applicability of allylhydrazine, a continuous counter-current back-extraction test of Np(VI) has been carried out using a miniature mixer-settler that consists of two steps: U-Pu recovery (3 stages) and Np separation (4 stages). Experimental results show that at least 90% of Np in feed are back-extracted and separated from U and Pu, therefore, it is confirmed that allylhydrazine is expected to be a selective salt-free reductant of Np(VI).
 doped with neptunium
doped with neptuniumSato, Tsuyoshi*; Yamashita, Toshiyuki; Matsui, Tsuneo*
Journal of Nuclear Materials, 344(1-3), p.67 - 72, 2005/09
Times Cited Count:4 Percentile:29.34(Materials Science, Multidisciplinary)Phase relationships between NpO and CaTiO
and CaTiO or Ca(Ti, Al)O
or Ca(Ti, Al)O were investigated by X-ray diffraction (XRD) analysis, using specimens prepared at 1773 K in Ar-8%H
were investigated by X-ray diffraction (XRD) analysis, using specimens prepared at 1773 K in Ar-8%H . Single phase solid solutions were formed 0-7.5 mol%Np and 1-10 mol%Np for CaTiO
. Single phase solid solutions were formed 0-7.5 mol%Np and 1-10 mol%Np for CaTiO and Ca(Ti, Al)O
and Ca(Ti, Al)O , respectively. By substituting Al for Ti in CaTiO
, respectively. By substituting Al for Ti in CaTiO , Np solubility in Ca(Ti, Al)O
, Np solubility in Ca(Ti, Al)O increased. Solubility of Np was compared with those of U and Pu, and was discussed with oxidation states and ionic radii of dopants. Thermal expansions of (Ca,Np)TiO
increased. Solubility of Np was compared with those of U and Pu, and was discussed with oxidation states and ionic radii of dopants. Thermal expansions of (Ca,Np)TiO were measured from room temperature to 1273 K in Ar-8%H
were measured from room temperature to 1273 K in Ar-8%H using high-temperature XRD technique. These specimens showed nearly the same value of volumetric thermal expansion coefficients, suggesting that the incorporation of tetravalent Np into CaTiO
using high-temperature XRD technique. These specimens showed nearly the same value of volumetric thermal expansion coefficients, suggesting that the incorporation of tetravalent Np into CaTiO had practically no effect on stabilization of the crystal lattice. This finding was in a marked contrast to that of Pu doped CaTiO
had practically no effect on stabilization of the crystal lattice. This finding was in a marked contrast to that of Pu doped CaTiO , where pronounced stabilization in the crystal was observed by incorporating Pu into CaTiO
, where pronounced stabilization in the crystal was observed by incorporating Pu into CaTiO .
.
 -butyraldehyde in tributyl phosphate diluted with
-butyraldehyde in tributyl phosphate diluted with  -dodecane
-dodecaneBan, Yasutoshi; Asakura, Toshihide; Morita, Yasuji
Radiochimica Acta, 92(12), p.883 - 887, 2004/12
Times Cited Count:13 Percentile:62.40(Chemistry, Inorganic & Nuclear)The reduction kinetics of Np(VI) by  -butyraldehyde in 30%TBP solution diluted with
-butyraldehyde in 30%TBP solution diluted with  -dodecane were analyzed by spectrophotometry. Based on the results of both
-dodecane were analyzed by spectrophotometry. Based on the results of both  -butyraldehyde and nitric acid concentration dependencies on the Np(VI) reduction reaction, the rate equation was obtained as -d
-butyraldehyde and nitric acid concentration dependencies on the Np(VI) reduction reaction, the rate equation was obtained as -d [Np(VI)]/d
[Np(VI)]/d =
= 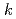 [
[ -C
-C H
H CHO]
CHO]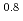 [HNO
[HNO ]
]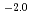 [Np(VI)]
[Np(VI)] where
where 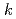 = (1.0
= (1.0 0.2)
0.2)  10
10 M
M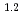 min
min at 294
at 294  1 K. The activation energy of the reaction was 76
1 K. The activation energy of the reaction was 76  5 kJ/mol. Redox equilibrium between Np(V) and N(VI), and reaction mechanism were discussed.
5 kJ/mol. Redox equilibrium between Np(V) and N(VI), and reaction mechanism were discussed.
Yamaguchi, Tetsuji; Nakayama, Shinichi; Yoshida, Takahiro
Radiochimica Acta, 92(9-11), p.677 - 682, 2004/12
Times Cited Count:6 Percentile:39.33(Chemistry, Inorganic & Nuclear)Adsorption of actinides onto negatively charged mineral surfaces was investigated under conditions that actinides were predominantly present as anionic complex species: Th(CO )
)
 , Am(CO
, Am(CO )
)
 , Np(CO
, Np(CO )
) (OH)
(OH)
 , UO
, UO (OH)
(OH)
 , NpO
, NpO (OH)
(OH)
 , Sn(OH)
, Sn(OH)
 and Pb(OH)
and Pb(OH)
 . These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,
. These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,  -Al
-Al O
O or SiO
or SiO (AEROSIL, specific surface area: 10
(AEROSIL, specific surface area: 10 m
m kg
kg ). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10
). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10 -molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25
-molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25 C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Uchiyama, Gunzo*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 262(2), p.311 - 315, 2004/11
Times Cited Count:11 Percentile:57.09(Chemistry, Analytical)no abstracts in English
 /Np couple at liquid Cd and Bi electrodes in LiCl-KCl eutectic melts
/Np couple at liquid Cd and Bi electrodes in LiCl-KCl eutectic meltsShirai, Osamu; Uozumi, Koichi*; Iwai, Takashi; Arai, Yasuo
Journal of Applied Electrochemistry, 34(3), p.323 - 330, 2004/03
Times Cited Count:29 Percentile:52.53(Electrochemistry)The electrode reactions of the Np /Np couple at liquid Cd and Bi electrodes were investigated by cyclic voltammetry at 723, 773 and 823 K in LiCl-KCl eutectic melt. It was found that the diffusion of Np
/Np couple at liquid Cd and Bi electrodes were investigated by cyclic voltammetry at 723, 773 and 823 K in LiCl-KCl eutectic melt. It was found that the diffusion of Np in the salt phase was a rate-determining step in the cathodic reaction when the concentration of NpCl
in the salt phase was a rate-determining step in the cathodic reaction when the concentration of NpCl was less than about 1 wt.% and the liquid Cd or Bi phase was not saturated with Np. The redox potentials of the Np
was less than about 1 wt.% and the liquid Cd or Bi phase was not saturated with Np. The redox potentials of the Np /Np couple at liquid Cd electrode at 723, 773 and 823 K were observed more positively than those at Mo electrode by 0.158, 0.140 and 0.126 V, respectively. The potential shift would result from a lowering of activity of Np in Cd phase according to the alloy formation of NpCd
/Np couple at liquid Cd electrode at 723, 773 and 823 K were observed more positively than those at Mo electrode by 0.158, 0.140 and 0.126 V, respectively. The potential shift would result from a lowering of activity of Np in Cd phase according to the alloy formation of NpCd at 723 K and NpCd
at 723 K and NpCd at 773 and 823 K. The redox potentials of the Np
at 773 and 823 K. The redox potentials of the Np /Np couple at liquid Bi electrode at 723, 773 and 823 K were more positive than those at Mo electrode by 0.427, 0.419 and 0.410 V, respectively, which would be attributable to a lowering of activity of Np in Bi phase according to the formation of NpBi
/Np couple at liquid Bi electrode at 723, 773 and 823 K were more positive than those at Mo electrode by 0.427, 0.419 and 0.410 V, respectively, which would be attributable to a lowering of activity of Np in Bi phase according to the formation of NpBi .
.
Saeki, Masakatsu
JAERI-Review 2003-030, 50 Pages, 2003/11
In this review, synthetic methods of uranium and neptunium compounds mainly from solutions and their natures are summarized. For uranium, 3 compounds of trivalent, 4 compounds of tetravalent and 23 compounds of hexavalent are described in slight details. For neptunium, synthetic methods of stock solutions for pentavalent and hexavalent neptunium, 4 items (6 compounds) of trivalent, 8 items (19 compounds) of tetravalent, 28 items (29 compounds) of pentavalent, 10 items (14 compounds) of hexavalent and 5 items (9 compounds) of heptavalent are described in slight details. Furthermore, on compounds which can not be described in details, the names are listed with literatures. The materials used here have been collected during the research activities on Moessbauer spectroscopic studies of uranium and neptunium compounds, then, the reviews on Moessbauer spectroscopic studies of actinoid compounds are also listed.
 Np and
Np and  Am through loess media
Am through loess mediaTanaka, Tadao; Mukai, Masayuki; Maeda, Toshikatsu; Matsumoto, Junko; Ogawa, Hiromichi; Li, Z.*; Wang, X.*; Fan, Z.*; Guo, L.*; Liu, C.*
Journal of Radioanalytical and Nuclear Chemistry, 256(2), p.205 - 211, 2003/05
Times Cited Count:3 Percentile:25.11(Chemistry, Analytical)Migration experiments of  Np(V) and
Np(V) and  Am(III) have been performed using a column system, to investigate migration behavior of Np and Am through a column packed with loess, taken from Shanxi, China. Adsorption mechanisms of Np and Am on the loess were examined by a chemical extraction method. In the case of the Np, most of Np adsorbed on the influent edge of the column. The Np adsorbed on the loess was mainly controlled by surface complexation. However, the migration of Np in the loess media could be roughly evaluated by using the distribution coefficient. In the case of the Am, particulate Am species was formed in the influent solution and moved in the column. The Am adsorbed on the loess was controlled by irreversible reactions. The migration behavior of particulate Am in the loess media could be expressed by the filtration theory.
Am(III) have been performed using a column system, to investigate migration behavior of Np and Am through a column packed with loess, taken from Shanxi, China. Adsorption mechanisms of Np and Am on the loess were examined by a chemical extraction method. In the case of the Np, most of Np adsorbed on the influent edge of the column. The Np adsorbed on the loess was mainly controlled by surface complexation. However, the migration of Np in the loess media could be roughly evaluated by using the distribution coefficient. In the case of the Am, particulate Am species was formed in the influent solution and moved in the column. The Am adsorbed on the loess was controlled by irreversible reactions. The migration behavior of particulate Am in the loess media could be expressed by the filtration theory.