Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hirade, Tetsuya
JPS Conference Proceedings (Internet), 25, p.011021_1 - 011021_2, 2019/03
Irradiation of water produce some reactive species such as OH radical. OH is formed by the reaction of a water cation and a water molecule just after ionization. On the other hand, a high energy positron injected in water will form cations and excess electrons even at the end part of the track. And hence, some positrons can form Positronium (Ps) with one of the excess electrons. The electrons in OH and Ps used to be in a same orbital in a water molecule before ionization of that water molecule. Therefore they were singlet at the time of the ionization. Every electron have each own hyperfine coupling constant after ionization. In water, reaction between Ps and OH, such as radical reaction or spin conversion, is possible. Therefore, quantum beats on these reaction can occur and the frequency of quantum beats will indicate the hyperfine coupling constant of OH which depends on the structure around OH. Therefore it is becoming possible to discuss the structure of water and reactivity of OH in the structure.
Hirade, Tetsuya
JPS Conference Proceedings (Internet), 25, p.011022_1 - 011022_3, 2019/03
OH radicals which are very reactive are formed by radiation decomposition in water. The behavior of OH radicals is important in corrosion of materials and reactions in living bodies. Recently, the reaction occurring between positronium (Ps) formed by OH radicals formed at the end of the positron track when positron is incident and positronium (Ps) formed by reaction of excess electrons formed with OH radical formation with the thermo-positron, it is reported that quantum beat occurs due to spin correlation. This quantum beat seems to have a period depending on the hyperfine coupling constant of OH radical. It is thought that the period and intensity of the quantum beat depends on the temperature, and it seems that it reflects the state around the OH radical. From the temperature dependence of the quantum beat detected by the reaction of this spin-correlated OH radical and triplet positronium we will explain what the liquid structure might be.
Taguchi, Mitsumasa; Kojima, Takuji
Radiation Research, 163(4), p.455 - 461, 2005/04
Times Cited Count:25 Percentile:57.33(Biology)no abstracts in English
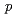 -nonylphenols in water by
-nonylphenols in water by  Co
Co  -ray irradiation
-ray irradiationKimura, Atsushi; Taguchi, Mitsumasa; Kojima, Takuji; Hiratsuka, Hiroshi*; Namba, Hideki
JAERI-Research 2004-018, 49 Pages, 2005/01
no abstracts in English
Taguchi, Mitsumasa; Kojima, Takuji
JAERI-Review 2004-025, TIARA Annual Report 2003, p.139 - 140, 2004/11
no abstracts in English
Taguchi, Mitsumasa; Kojima, Takuji
JAERI-Review 2004-025, TIARA Annual Report 2003, p.141 - 142, 2004/11
no abstracts in English
Taguchi, Mitsumasa; Kojima, Takuji
JAERI-Review 2003-033, TIARA Annual Report 2002, p.141 - 142, 2003/11
no abstracts in English
Yoshida, Yoichi*; Yang, J.*; Seki, Shuhei*; Saeki, Akinori*; Tagawa, Seiichi*; Shibata, Hiromi*; Taguchi, Mitsumasa; Kojima, Takuji; Namba, Hideki
JAERI-Review 2003-033, TIARA Annual Report 2002, p.145 - 146, 2003/11
no abstracts in English
 -ray irradiation
-ray irradiationAbe, Yasuhiro*; Takigami, Machiko; Sugino, Koji*; Taguchi, Mitsumasa; Kojima, Takuji; Umemura, Tomonari*; Tsunoda, Kinichi*
Bulletin of the Chemical Society of Japan, 76(8), p.1681 - 1685, 2003/08
Times Cited Count:5 Percentile:26.45(Chemistry, Multidisciplinary)The decomposition of phenolic endocrine disrupting chemicals (P-EDCs), such as phenol, 4-butylphenol (BuP), and bisphenol A (BPA), in aqueous solutions by potassium permanganate (KMnO ) was studied and its efficiency was compared with that of hydroxyl radicals (OH
) was studied and its efficiency was compared with that of hydroxyl radicals (OH ) generated by
) generated by  Co
Co  -ray irradiation. Various organic acids and inorganic carbon were formed in the decomposition of P-EDCs due to either KMnO
-ray irradiation. Various organic acids and inorganic carbon were formed in the decomposition of P-EDCs due to either KMnO or OH
or OH . They were formed via direct aromatic ring cleavage in the case of KMnO
. They were formed via direct aromatic ring cleavage in the case of KMnO and OH
and OH addition-substitution reactions followed by aromatic ring cleavage in the case of OH
addition-substitution reactions followed by aromatic ring cleavage in the case of OH . Comparing the decrease in the P-EDCs based on the number of electrons, the amount of KMnO
. Comparing the decrease in the P-EDCs based on the number of electrons, the amount of KMnO spent to completely eliminate BuP and BPA was comparable to the amount of OH
spent to completely eliminate BuP and BPA was comparable to the amount of OH . Although three times more KMnO
. Although three times more KMnO was needed for phenol than OH
was needed for phenol than OH , the complete conversion of phenol into organic acids and inorganic carbon was achieved with 720
, the complete conversion of phenol into organic acids and inorganic carbon was achieved with 720 M of electrons in both cases.
M of electrons in both cases.
Kim, H.*; Hakoda, Teruyuki; Kojima, Takuji
Journal of Physics D; Applied Physics, 36(5), p.473 - 481, 2003/03
Times Cited Count:6 Percentile:28.14(Physics, Applied)no abstracts in English
Watanabe, Ritsuko; Saito, Kimiaki
Radiation Physics and Chemistry, 62(2-3), p.217 - 228, 2001/09
Times Cited Count:36 Percentile:90.74(Chemistry, Physical)no abstracts in English

 and H
and H O
O in oxygen effect of pseudomonas radiora O-1
in oxygen effect of pseudomonas radiora O-1; *; Takehisa, Masaaki
Shokuhin Shosha, 17, p.20 - 22, 1982/00
no abstracts in English
; ; *; *
Agricultural and Biological Chemistry, 45(6), p.1311 - 1315, 1981/00
no abstracts in English
 O in aqueous suspension
O in aqueous suspension; *; Takehisa, Masaaki
Radiat.Res., 88(3), p.577 - 586, 1981/00
Times Cited Count:27 Percentile:77.47(Biology)no abstracts in English
; *; Takehisa, Masaaki
Z.Allg.Mikrobiol., 20(8), p.535 - 537, 1980/00
no abstracts in English
 , He
, He
 , C
, C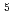
 and Ne
and Ne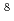
 ions
ionsIwamatsu, Kazuhiro; Yamashita, Shinichi*; Taguchi, Mitsumasa; Kimura, Atsushi; Kurashima, Satoshi; Katsumura, Yosuke
no journal, ,
Heavy ion beams, one of the high linear energy transfer (LET) radiations induce specific irradiation effects which are different from those of low LET radiations. The effects are attributed to heterogeneous distribution of reactive species along their trajectories, so called "track structure". Water was selected as target in this study because more data exist for radiolysis than any other substances. Hydroxyl radical (OH), one of the most important water decomposition species, was focused on by using bromide ion as a probing reagent, and their reactions were observed by the ion beam pulse radiolysis system. The formation and decay of Br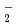 were observed at 375 nm (
were observed at 375 nm ( [Br
[Br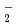 ] = 9000 M
] = 9000 M
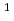 cm
cm
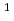 ). The formation chemical yield of it after pulse irradiation decreased with increasing atomic numbers of the incident ions. Radical recombination reaction occurs easier by higher LET ions because of denser radical formations. Therefore, the decrease in the yields of OH scavenged by Br
). The formation chemical yield of it after pulse irradiation decreased with increasing atomic numbers of the incident ions. Radical recombination reaction occurs easier by higher LET ions because of denser radical formations. Therefore, the decrease in the yields of OH scavenged by Br induces the decrease in the chemical yields of Br
induces the decrease in the chemical yields of Br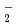 . The decay rates of the chemical yields increased with the atomic mass of the ions. The concentrations depend on the dose rate and chemical yield, and the dose rates proportional to LET value. The concentrations after pulse increased with increase in the atomic mass of the ions and resulted in faster decay in heavier ions.
. The decay rates of the chemical yields increased with the atomic mass of the ions. The concentrations depend on the dose rate and chemical yield, and the dose rates proportional to LET value. The concentrations after pulse increased with increase in the atomic mass of the ions and resulted in faster decay in heavier ions.
Hirade, Tetsuya
no journal, ,
A high energy positron injected in water will form cations and then OH radical will be formed. On the other hand, an excess electron formed by the inization and positrons can form Positronium (Ps). The electrons in OH and Ps used to be in a same orbital in a water molecule before ionization of that water molecule. Therefore they were singlet at the time of the ionization. Every electron have each own hyperfine coupling constant after ionization. In water, reaction between Ps and OH, such as radical reaction or spin conversion, is possible. Therefore, quantum beats on these reaction can occur and the frequency of quantum beats will indicate the hyperfine coupling constant of OH which depends on the structure around OH. At 15 C and 10
C and 10 C, quantum beats were observed and it indicated that water structure changed at these temperature range.
C, quantum beats were observed and it indicated that water structure changed at these temperature range.
Hirade, Tetsuya
no journal, ,
The annihilation lifetime of triplet Ps (o-Ps) in water observed by the positron annihilation lifetime (PALS) measurement shows opposite temperature dependence of other usual liquids, i.e. shorter lifetimes at higher temperatures. This is because o-Ps reacts with surrounding active species in water. In the positron annihilation lifetime-momentum correlation (AMOC) measurement, in particular, singlet Ps (p-Ps) formed by spin exchange reaction is detected effectively, and it is predicted that temperature dependence is observed like PALS measurement. In fact, above about 10 C, the formation of p-Ps by the spin exchange reaction decreased with lowering temperatures. However, at around 6
C, the formation of p-Ps by the spin exchange reaction decreased with lowering temperatures. However, at around 6 C, the formation of p-Ps by the spin exchange reaction increased again. The reason of this phenomenon is that the reaction with the OH radical with the electron having a spin correlation with the electron in o-Ps preferentially occurs. Hence, three-dimensional diffusion dominates the reaction above 10
C, the formation of p-Ps by the spin exchange reaction increased again. The reason of this phenomenon is that the reaction with the OH radical with the electron having a spin correlation with the electron in o-Ps preferentially occurs. Hence, three-dimensional diffusion dominates the reaction above 10 C and OH radical on the surface of water cluster preferentially reacts with o-Ps below 10
C and OH radical on the surface of water cluster preferentially reacts with o-Ps below 10 C.
C.
Hirade, Tetsuya
no journal, ,
A positron injected in water ionizes a water molecule at the end of the positron track and a cation and an excess electron will be formed. The injected positron and the excess electron will form ortho-positronium (o-Ps) that has a long lifetime (several nanoseconds) with a certain probability. The cation immediately becomes an OH radical. Therefore, the o-Ps and the OH radical formed within a short distance and have an electron-spin correlation. The effect of the spin correlation of the reaction between these spin-correlated o-Ps and OH radical indicated that long-range diffusion of OH radicals could be suppressed in the presence of water clusters.
Hirade, Tetsuya
no journal, ,
The behavior of OH radicals formed by radiolysis of water is important for reactions in living organisms and corrosion in nuclear reactors. However, OH radicals in various environments exist in the complex structure in water. By utilizing the positron annihilation lifetime-momentum correlation (AMOC), when OH radical having a spin correlation with an electron in the Ps formed at the end of the track reacts with the long-lived ortho-Ps, it can be identified from the yield of singlet Ps after the spin-exchange reaction is larger than that of OH radicals having no spin correlation. AMOC measurements showed that the diffusion behavior change of OH radicals was probably caused by the change of the water liquid structure in the temperature range of 10 to 15  C and 1 to 2
C and 1 to 2  C.
C.