Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Teraoka, Yuden
Denki Gakkai Gijutsu Hokoku, (970), p.10 - 15, 2004/07
Si(001) surfaces are oxidized by O molecules. The reaction schemes (oxide-layers formation, SiO desorption, their coexistence) are changed depending on the surface temperature and the gas pressure. The translational kinetic energy of incident O
molecules. The reaction schemes (oxide-layers formation, SiO desorption, their coexistence) are changed depending on the surface temperature and the gas pressure. The translational kinetic energy of incident O molecules is recognizing to be an important parameter for controlling surface chemical reactions. The issues concerning translational kinetic energy induced oxidation by O
molecules is recognizing to be an important parameter for controlling surface chemical reactions. The issues concerning translational kinetic energy induced oxidation by O molecules at room temperature, effects of translational kinetic energy for SiO desorption processes at higher temperature than 1000 K, reaction mechanisms for coexistence of the SiO desorption and the oxide-layers formation in the temperature region from 900 K to 1000 K are reviewed.
molecules at room temperature, effects of translational kinetic energy for SiO desorption processes at higher temperature than 1000 K, reaction mechanisms for coexistence of the SiO desorption and the oxide-layers formation in the temperature region from 900 K to 1000 K are reviewed.
Ogawa, Shuichi*; Takakuwa, Yuji*; Ishizuka, Shinji*; Mizuno, Yoshiyuki*; Tonda, Hideki*; Homma, Teiichi*; Teraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke; Hachiue, Shunsuke
JAERI-Tech 2004-046, 25 Pages, 2004/06
We investigated the correlation between initial sticking coefficient and O transitional kinetic energy to understand O
transitional kinetic energy to understand O adsorption processes on the Ti(0001) surface via photoemission spectroscopy for O-1s and Ti-2p core levels using the surface reaction analysis apparatus, installed at the JAERI soft X-ray beamline BL23SU in the SPring-8. We observed the decrease of initial sticking coefficient of O
adsorption processes on the Ti(0001) surface via photoemission spectroscopy for O-1s and Ti-2p core levels using the surface reaction analysis apparatus, installed at the JAERI soft X-ray beamline BL23SU in the SPring-8. We observed the decrease of initial sticking coefficient of O molecules on the Ti(0001) surface with increasing O
molecules on the Ti(0001) surface with increasing O transitional kinetic energy. We concluded that the O
transitional kinetic energy. We concluded that the O adsorption on the Ti(0001) surface proceeded by a trapping-mediated dissociative adsorption mechanism. The constant dependence of the initial sticking coefficient on incident angle of O
adsorption on the Ti(0001) surface proceeded by a trapping-mediated dissociative adsorption mechanism. The constant dependence of the initial sticking coefficient on incident angle of O beams also suggested the propriety of the trapping-mediated surface reaction mechanism.
beams also suggested the propriety of the trapping-mediated surface reaction mechanism.
Teraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke
Shinku, 47(4), p.301 - 307, 2004/04
Recent research results on translational kinetic energy effects of incident oxygen molecules for Si(001) oxidation are summalized and introduced. The variation of surface temperature dependence of SiO desorption yield, oxygen uptake curves, and chemical bonding states depending on translational kinetic energy of oxygen molecules is described concretely. Eapecially, the translational kinetic energy effects on chemical reaction processes of concurrent oxide-layers formation and SiO desorption are discussed.
 reaction dynamics with Si(001) surfaces as observed by synchrotron radiation photoemission spectroscopy
reaction dynamics with Si(001) surfaces as observed by synchrotron radiation photoemission spectroscopyTeraoka, Yuden; Yoshigoe, Akitaka
Atomic Collision Research in Japan, No.28, p.97 - 99, 2002/00
The translational kinetic energy of incident molecules is an important factor for the induction of surface reactions. We applied supersonic seed molecular beam techniques and high-energy-resolution photoemission spectroscopy using synchrotron radiation to the Si initial oxidation analysis. We have already found out that the saturated oxygen coverage on H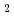 O-chemisorbed Si(001) surfaces depends on the O
O-chemisorbed Si(001) surfaces depends on the O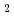 incident energy. Two potential energy barriers were confirmed in accordance with the first-principles calculation. An action of the incident energy should be confirmed also on clean Si(001) surfaces. Therefore, the incident energy dependence of the O
incident energy. Two potential energy barriers were confirmed in accordance with the first-principles calculation. An action of the incident energy should be confirmed also on clean Si(001) surfaces. Therefore, the incident energy dependence of the O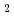 dissociative chemisorption on the clean Si(001) surface has been investigated by photoemission spectroscopy for Si-2p and O-1s core levels to make clear how the incident energy affects the ultra-thin oxide-layers formation.
dissociative chemisorption on the clean Si(001) surface has been investigated by photoemission spectroscopy for Si-2p and O-1s core levels to make clear how the incident energy affects the ultra-thin oxide-layers formation.
 analysis using high resolution synchrotron radiation photoemission spectroscopy for initial oxidation of H
analysis using high resolution synchrotron radiation photoemission spectroscopy for initial oxidation of H O pre-adsorbed Si(001) surfaces induced by supersonic O
O pre-adsorbed Si(001) surfaces induced by supersonic O molecular beams at room temperature
molecular beams at room temperatureYoshigoe, Akitaka; Teraoka, Yuden
Atomic Collision Research in Japan, No.28, p.105 - 107, 2002/00
The oxidation states up to Si states were formed when the translational kinetic energy of O
states were formed when the translational kinetic energy of O was 3.0 eV. The time evolustions of each Si oxidation states were in situ measured by using the high resolution photoemission spectroscopy.The translational kinetic energy of 3.0eV randomly enhanced the oxidation on Si(001) surface up to the second layer Si backbonds. It was found that the Si
was 3.0 eV. The time evolustions of each Si oxidation states were in situ measured by using the high resolution photoemission spectroscopy.The translational kinetic energy of 3.0eV randomly enhanced the oxidation on Si(001) surface up to the second layer Si backbonds. It was found that the Si was not observed, thus the dimer Si atoms at the top layer was not surrounded by four oxygen atoms at the initial oxidation stage.
was not observed, thus the dimer Si atoms at the top layer was not surrounded by four oxygen atoms at the initial oxidation stage.
 -O
-O -alkane(C
-alkane(C -C
-C ) mixtures studied by time-resolved atmospheric pressure ionization mass spectrometry
) mixtures studied by time-resolved atmospheric pressure ionization mass spectrometry; Ikezoe, Yasumasa
J.Phys.Chem., 92(5), p.1126 - 1133, 1988/05
no abstracts in English