Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
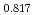 Pu
Pu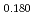 Am
Am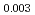 O
O uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 K
uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 KVauchy, R.; Sunaoshi, Takeo*; Hirooka, Shun; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 580, p.154416_1 - 154416_11, 2023/07
Times Cited Count:4 Percentile:96.18(Materials Science, Multidisciplinary)
Watanabe, Masashi; Kato, Masato
Frontiers in Nuclear Engineering (Internet), 1, p.1082324_1 - 1082324_9, 2023/01
Since the oxygen potential and the oxygen coefficient of UO have a significant impact on fuel performance, many experimental data have been obtained. However, experimental data of the oxygen potential and the oxygen diffusion coefficient in the high temperature region above 1673 K are very limited. In the present study, we aimed to obtain these data and analyze them by defect chemistry. The oxygen potentials and the oxygen chemical diffusion coefficient of UO
have a significant impact on fuel performance, many experimental data have been obtained. However, experimental data of the oxygen potential and the oxygen diffusion coefficient in the high temperature region above 1673 K are very limited. In the present study, we aimed to obtain these data and analyze them by defect chemistry. The oxygen potentials and the oxygen chemical diffusion coefficient of UO were measured by the gas equilibrium method in the near stoichiometric region at temperatures ranging from 1673 to 1873 K. A data set of oxygen potentials was made together with literature data and analyzed by defect chemistry. The oxygen potential of UO
were measured by the gas equilibrium method in the near stoichiometric region at temperatures ranging from 1673 to 1873 K. A data set of oxygen potentials was made together with literature data and analyzed by defect chemistry. The oxygen potential of UO was determined as a function of O/U ratio and temperature, and an equation representing the relationship was derived. The oxygen chemical diffusion coefficient values obtained in this study were reasonably close to the literature values. The oxygen partial pressure dependence of the oxygen chemical diffusion coefficients was predicted from the evaluated results of the oxygen potential data, but no clear dependence was observed.
was determined as a function of O/U ratio and temperature, and an equation representing the relationship was derived. The oxygen chemical diffusion coefficient values obtained in this study were reasonably close to the literature values. The oxygen partial pressure dependence of the oxygen chemical diffusion coefficients was predicted from the evaluated results of the oxygen potential data, but no clear dependence was observed.
 and (U,Pu,Am,Np)O
and (U,Pu,Am,Np)O
Hirooka, Shun; Matsumoto, Taku; Kato, Masato; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*
Journal of Nuclear Materials, 542, p.152424_1 - 152424_9, 2020/12
Times Cited Count:6 Percentile:59.07(Materials Science, Multidisciplinary)The measurement of oxygen potential was conducted at 1,673, 1,773, and 1,873 K for (U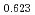 Pu
Pu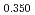 Am
Am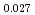 )O
)O and at 1,873 and 1,923 K for (U
and at 1,873 and 1,923 K for (U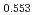 Pu
Pu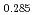 Am
Am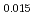 Np
Np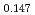 )O
)O by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO
by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
 Zr
Zr O
O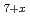
Otobe, Haruyoshi; Nakamura, Akio; Yamashita, Toshiyuki; Minato, Kazuo
Journal of Physics and Chemistry of Solids, 66(2-4), p.329 - 334, 2005/02
Times Cited Count:23 Percentile:65.8(Chemistry, Multidisciplinary)Pyrochlore-type (P-type) zirconias have been attracting significant research interest as disposable forms of high-level radioactive waste. In this work, we have clarified the oxygen potentials (g(O )) vs. oxygen nonstoichiometry (x) and temperature relations of P-type Ce
)) vs. oxygen nonstoichiometry (x) and temperature relations of P-type Ce Zr
Zr O
O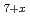 by the emf method and determined the lattice parameters (a0) with respect to x by XRD method.
by the emf method and determined the lattice parameters (a0) with respect to x by XRD method.

Fujino, Takeo*; Sato, Nobuaki*; Yamada, Kota*; Okazaki, Manabu*; Fukuda, Kosaku; Serizawa, Hiroyuki; Shiratori, Tetsuo*
Journal of Nuclear Materials, 289(3), p.270 - 280, 2001/03
Times Cited Count:2 Percentile:19.6(Materials Science, Multidisciplinary)no abstracts in English
A.Li*; H.Huang*; D.Li*; S.Zheng*; H.Du*; S.Zhu*; Iwata, Tadao
Japanese Journal of Applied Physics, 32(3), p.1033 - 1038, 1993/03
Times Cited Count:8 Percentile:44.65(Physics, Applied)no abstracts in English
; H.Gnaser*; W.O.Hofer*
Nuclear Instruments and Methods in Physics Research B, 28, p.540 - 547, 1987/00
Times Cited Count:11 Percentile:76.82(Instruments & Instrumentation)no abstracts in English


 at relatively small deviation from stoichiometry between 600 and 1400
at relatively small deviation from stoichiometry between 600 and 1400 C
CNakamura, Akio; Fujino, Takeo
Journal of Nuclear Materials, 140, p.113 - 130, 1986/09
Times Cited Count:17 Percentile:83.67(Materials Science, Multidisciplinary)no abstracts in English
 ; Electronic structure of the hydrogen-like defects and their potential use in depth-resolved detection of oxygen vacancies
; Electronic structure of the hydrogen-like defects and their potential use in depth-resolved detection of oxygen vacanciesIto, Takashi; Higemoto, Wataru; Koda, Akihiro*; Shimomura, Koichiro*
no journal, ,
 as a function of Pu content
as a function of Pu contentHirooka, Shun; Matsumoto, Taku; Morimoto, Kyoichi; Ogasawara, Masahiro*; Murakami, Tatsutoshi; Kato, Masato
no journal, ,
no abstracts in English