Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Teshigawara, Makoto; Lee, Y.*; Tatsumoto, Hideki*; Hartl, M.*; Aso, Tomokazu; Iverson, E. B.*; Ariyoshi, Gen; Ikeda, Yujiro*; Hasegawa, Takumi*
Nuclear Instruments and Methods in Physics Research B, 557, p.165534_1 - 165534_10, 2024/12
Times Cited Count:1 Percentile:0.00(Instruments & Instrumentation)At Japanese Spallation Neutron Source in J-PARC, the para-hydrogen fraction was measured by using Raman spectroscopy in-situ for an integrated beam power of 9.4 MW h at 1 MW operation, to evaluate the functionality of the ferric oxyhydroxide catalyst. This result showed that full functionality of the catalyst was retained up to the 1 MW operation. We attempted to study the effect of neutron scattering driven para to ortho-hydrogen back-conversion rate in the absence of the catalyst effect with a bypass line without catalyst. The measured increase of ortho-hydrogen fraction was 0.44% for an integrated beam power of 2.4 MW
h at 1 MW operation, to evaluate the functionality of the ferric oxyhydroxide catalyst. This result showed that full functionality of the catalyst was retained up to the 1 MW operation. We attempted to study the effect of neutron scattering driven para to ortho-hydrogen back-conversion rate in the absence of the catalyst effect with a bypass line without catalyst. The measured increase of ortho-hydrogen fraction was 0.44% for an integrated beam power of 2.4 MW h at 500 kW operation, however, which was considered to be due to not only to neutron collisions in cold moderators but also to the high ortho-hydrogen fraction of initially static liquid hydrogen in the bypass line and passive exudation of quasi-static hydrogen in the catalyst vessel to the main loop.
h at 500 kW operation, however, which was considered to be due to not only to neutron collisions in cold moderators but also to the high ortho-hydrogen fraction of initially static liquid hydrogen in the bypass line and passive exudation of quasi-static hydrogen in the catalyst vessel to the main loop.

McGrady, J.; Kumagai, Yuta; Watanabe, Masayuki; Kirishima, Akira*; Akiyama, Daisuke*; Kimuro, Shingo; Ishidera, Takamitsu
Journal of Nuclear Science and Technology, 60(12), p.1586 - 1594, 2023/12
Times Cited Count:2 Percentile:43.92(Nuclear Science & Technology) and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:4 Percentile:51.78(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U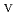
 Fe
Fe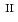
 U
U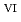 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
Arai, Yosuke*; Kuroda, Kenta*; Nomoto, Takuya*; Tin, Z. H.*; Sakuragi, Shunsuke*; Bareille, C.*; Akebi, Shuntaro*; Kurokawa, Kifu*; Kinoshita, Yuto*; Zhang, W.-L.*; et al.
Nature Materials, 21(4), p.410 - 415, 2022/04
Times Cited Count:14 Percentile:76.79(Chemistry, Physical)Saeki, Morihisa*; Yomogida, Takumi; Matsumura, Daiju; Saito, Takumi*; Nakanishi, Ryuzo*; Tsuji, Takuya; Oba, Hironori*
Analytical Sciences, 36(11), p.1371 - 1378, 2020/11
Times Cited Count:6 Percentile:20.69(Chemistry, Analytical)We measured X-ray absorption fine structure (XAFS) and Raman spectra of isopolymolybdates(VI) in HNO solution (0.15- 4.0 M), which change their geometries depending on acid concentration, and performed simultaneous resolution of the XAFS and Raman data using a multivariate curve resolution by alternating least-squares (MCR-ALS) analysis. In iterative ALS optimization, initial data matrices were prepared by two different methods. The MCR-ALS result of single XAFS data matrix shows large dependence on the preparation method of the initial data matrices. The MCR-ALS result of an augmented matrix of Raman and XAFS data has little dependence on the initial data matrices. It indicates that the augmentation method effectively improves the rotation ambiguities in the MCR-ALS analysis of the XAFS data. Based on the model fitting of the pure EXAFS oscillations, we revealed the change of [Mo
solution (0.15- 4.0 M), which change their geometries depending on acid concentration, and performed simultaneous resolution of the XAFS and Raman data using a multivariate curve resolution by alternating least-squares (MCR-ALS) analysis. In iterative ALS optimization, initial data matrices were prepared by two different methods. The MCR-ALS result of single XAFS data matrix shows large dependence on the preparation method of the initial data matrices. The MCR-ALS result of an augmented matrix of Raman and XAFS data has little dependence on the initial data matrices. It indicates that the augmentation method effectively improves the rotation ambiguities in the MCR-ALS analysis of the XAFS data. Based on the model fitting of the pure EXAFS oscillations, we revealed the change of [Mo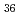 O
O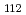 (H
(H O)
O)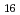 ]
]
 [Mo
[Mo O
O (H
(H O)
O) ]
]
 [HMoO
[HMoO (H
(H O)
O) ]
] in the highly concentrated HNO
in the highly concentrated HNO solution.
solution.
 thin films by ion irradiation
thin films by ion irradiationNarumi, Kazumasa; Sakai, Seiji; Naramoto, Hiroshi*; Takanashi, Koki
JAEA-Review 2005-001, TIARA Annual Report 2004, p.238 - 240, 2006/01
no abstracts in English
 films polymerized by ion irradiation
films polymerized by ion irradiationNarumi, Kazumasa; Sakai, Seiji; Naramoto, Hiroshi*; Takanashi, Koki
Fullerenes, Nanotubes, and Carbon Nanostructures, 14(2-3), p.429 - 434, 2006/00
no abstracts in English
 -irradiated diamond
-irradiated diamondXu, Y.; Naramoto, Hiroshi; Narumi, Kazumasa; Miyashita, Kiyoshi*; Kamiya, Tomihiro; Sakai, Takuro
Applied Physics Letters, 83(10), p.1968 - 1970, 2003/09
Times Cited Count:6 Percentile:27.75(Physics, Applied)no abstracts in English
Kato, Chiaki
JAERI-Research 2003-013, 143 Pages, 2003/08
This study is investigation about stress corrosion cracking (SCC) of zirconium in nuclear fuel reprocessing. Chapter 1 is described background. Chapter 2 is explained experimental apparates. Chapter 3 is described the increased oxidization potential on the heat-transfer surface and suggested the initiation of SCC on a boiling heat-transfer surface. Chapter 4 is described that the SCC susceptibility increased with increasing nitric acid concentration and solution temperature on notched specimen by SSRT. In addition, the SCC susceptibility effected by the crystal anisotropy by the hot rolling direction and increased on a parallel face to the rolling direction. Chapter 5 is described that the SCC susceptibility increased in HAZ/base metal boundary in order to the preferential orientation of cleavage plane (0002). Chapter 6 is described that the increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition on the surface and the removal of the decomposition product from solution by boiling bubbles.
Wei, P.; Xu, Y.; Nagata, Shinji*; Narumi, Kazumasa; Naramoto, Hiroshi
Nuclear Instruments and Methods in Physics Research B, 206(1-4), p.233 - 236, 2003/05
Times Cited Count:6 Percentile:42.61(Instruments & Instrumentation)no abstracts in English
 and Ni on the MgO(100) single crystal
and Ni on the MgO(100) single crystalVacik, J.; Naramoto, Hiroshi; Narumi, Kazumasa; Yamamoto, Shunya; Miyashita, Kiyoshi*
Materials Research Society Symposium Proceedings, Vol.648, p.P3.50.1 - P3.50.6, 2001/00
None
Zhang, Z.; Narumi, Kazumasa; Naramoto, Hiroshi; Wu, Z.*; Yamamoto, Shunya; Miyashita, Atsumi
Journal of Physics; Condensed Matter, 11(25), p.L273 - L277, 1999/06
Times Cited Count:3 Percentile:12.39(Physics, Condensed Matter)no abstracts in English
; Hayashi, Kimio; Fukuda, Kosaku
JAERI-M 92-114, 20 Pages, 1992/08
no abstracts in English
Igawa, Naoki; Ono, Hideo; Nagasaki, Takanori; Ishii, Yoshinobu; Noda, Kenji; Watanabe, H.; Matsuo, Toru*; Igarashi, Kazuo*
Ceramic Transactions, Vol.27, p.135 - 156, 1992/00
no abstracts in English
 -BeF
-BeF based fluoride glasses for IR transmission
based fluoride glasses for IR transmissionOno, Hideo; Igawa, Naoki; Ishii, Yoshinobu; Umesaki, Norimasa*;
Journal of Nuclear Materials, 191-194, p.525 - 529, 1992/00
no abstracts in English
Okuno, Kenji; ; Ohira, Shigeru; Naruse, Yuji
Journal of Nuclear Science and Technology, 28(6), p.509 - 516, 1991/06
no abstracts in English
; ; ; ; Ono, Hideo; Igawa, Naoki; Nagasaki, Takanori
Journal of the American Ceramic Society, 73(8), p.2523 - 2525, 1990/00
Times Cited Count:13 Percentile:53.85(Materials Science, Ceramics)no abstracts in English
Nomura, Shinzo; Saito, Tamotsu; Imai, Hisashi
Tanso, 0(140), p.275 - 281, 1989/00
no abstracts in English
J.Vac.Sci.Technol.,A, 5(4), p.2222 - 2226, 1987/04
no abstracts in English
Miro, S.*; Peuget, S.*; Tupin, M.*; Fayette, L.*; Nakayoshi, Akira; Perrin, S.*; Jegou, C.*
no journal, ,