Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Np,
Np,  Am, and
Am, and  Am reaction rates in highly enriched uranium fuel cores at Kyoto University Critical Assembly
Am reaction rates in highly enriched uranium fuel cores at Kyoto University Critical AssemblyPyeon, C. H.*; Oizumi, Akito; Katano, Ryota; Fukushima, Masahiro
Nuclear Science and Engineering, 199(3), p.429 - 444, 2025/03
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Experimental analyses of neptunium-237 ( Np), americium-241 (
Np), americium-241 ( Am), and
Am), and  Am fission and
Am fission and  Np capture reaction rates are conducted by the Serpent 2 code together with ENDF/B-VIII.0 and JENDL-5, using experimental data at neutron spectra of thermal and intermediate regions obtained in the solid-moderated and solid-reflected cores with highly-enriched uranium fuel at the Kyoto University Critical Assembly. Also, uncertainty quantification of fission and capture reaction rate ratios of test samples of
Np capture reaction rates are conducted by the Serpent 2 code together with ENDF/B-VIII.0 and JENDL-5, using experimental data at neutron spectra of thermal and intermediate regions obtained in the solid-moderated and solid-reflected cores with highly-enriched uranium fuel at the Kyoto University Critical Assembly. Also, uncertainty quantification of fission and capture reaction rate ratios of test samples of  Np,
Np,  Am and
Am and  Am with reference samples of uranium-235 (
Am with reference samples of uranium-235 ( U) and gold-197 (
U) and gold-197 (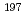 Au) are evaluated by the MARBLE code system. In terms of fission reaction rate ratios of
Au) are evaluated by the MARBLE code system. In terms of fission reaction rate ratios of  Np/
Np/ U,
U,  Am/
Am/ U and
U and  Am/
Am/ U, a comparison between experiments and Serpent 2 calculations shows an accuracy about 5, 15 and 10%, respectively, together with ENDF/B-VIII.0 and JENDL-5. For capture reaction rate ratios of
U, a comparison between experiments and Serpent 2 calculations shows an accuracy about 5, 15 and 10%, respectively, together with ENDF/B-VIII.0 and JENDL-5. For capture reaction rate ratios of  Np/
Np/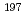 Au, Serpent 2 calculations reveal a fairly good accuracy at the thermal neutron spectrum. The total uncertainties of
Au, Serpent 2 calculations reveal a fairly good accuracy at the thermal neutron spectrum. The total uncertainties of  Np/
Np/ U,
U,  Am/
Am/ U and
U and  Am/
Am/ U fission reaction rate ratios by MARBLE with the covariance data of ENDF/B-VIII.0 and JENDL-5 are found to be about 4% at most in all cores, except for about 8% of
U fission reaction rate ratios by MARBLE with the covariance data of ENDF/B-VIII.0 and JENDL-5 are found to be about 4% at most in all cores, except for about 8% of  Am/
Am/ U with ENDF/B-VIII.0 at the intermediate neutron spectrum.
U with ENDF/B-VIII.0 at the intermediate neutron spectrum.
 dissolution in bicarbonate solution with H
dissolution in bicarbonate solution with H O
O ; The Effect of temperature
; The Effect of temperatureMcGrady, J.; Kumagai, Yuta; Kitatsuji, Yoshihiro; Kirishima, Akira*; Akiyama, Daisuke*; Watanabe, Masayuki
RSC Advances (Internet), 13(40), p.28021 - 28029, 2023/09
Times Cited Count:2 Percentile:21.35(Chemistry, Multidisciplinary)Upon nuclear waste canister failure and contact of spent nuclear fuel with groundwater, the UO matrix of spent fuel will interact with oxidants in the groundwater generated by water radiolysis. Bicarbonate (HCO
matrix of spent fuel will interact with oxidants in the groundwater generated by water radiolysis. Bicarbonate (HCO
 ) is often found in groundwater, and the H
) is often found in groundwater, and the H O
O induced oxidative dissolution of UO
induced oxidative dissolution of UO in bicarbonate solution has previously been studied under various conditions. Temperatures in the repository at the time of canister failure will differ depending on the location, yet the effect of temperature on oxidative dissolution is unknown. To investigate, the decomposition rate of H
in bicarbonate solution has previously been studied under various conditions. Temperatures in the repository at the time of canister failure will differ depending on the location, yet the effect of temperature on oxidative dissolution is unknown. To investigate, the decomposition rate of H O
O at the UO
at the UO surface and dissolution of U
surface and dissolution of U in bicarbonate solution (0.1, 1, 10 and 50 mM) was analysed at various temperatures (10, 25, 45 and 60
in bicarbonate solution (0.1, 1, 10 and 50 mM) was analysed at various temperatures (10, 25, 45 and 60  C). At [HCO
C). At [HCO
 ]
] 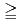 1 mM, the apparent equilibrium concentration of U
1 mM, the apparent equilibrium concentration of U decreased with increasing temperature. This was attributed to the formation of U
decreased with increasing temperature. This was attributed to the formation of U -bicarbonate species at the surface and a change in the mechanism of H
-bicarbonate species at the surface and a change in the mechanism of H O
O decomposition from oxidative to catalytic. At 0.1 mM, no obvious correlation between temperature and U dissolution was observed, and thermodynamic calculations indicated this was due to a change in the surface species. A pathway to explain the observed dissolution behaviour of UO
decomposition from oxidative to catalytic. At 0.1 mM, no obvious correlation between temperature and U dissolution was observed, and thermodynamic calculations indicated this was due to a change in the surface species. A pathway to explain the observed dissolution behaviour of UO in bicarbonate solution as a function of temperature was proposed.
in bicarbonate solution as a function of temperature was proposed.
Yamamoto, Tomohiko; Kato, Atsushi; Hayakawa, Masato; Shimoyama, Kazuhito; Ara, Kuniaki; Hatakeyama, Nozomu*; Yamauchi, Kanau*; Eda, Yuhei*; Yui, Masahiro*
Proceedings of 2023 International Congress on Advanced in Nuclear Power Plants (ICAPP 2023) (Internet), 6 Pages, 2023/04
Saito, Tatsuo; Yamazawa, Hiromi*; Mochizuki, Akihito
Journal of Environmental Radioactivity, 255, p.107035_1 - 107035_14, 2022/12
Times Cited Count:0 Percentile:0.00(Environmental Sciences)The seasonal variation of dissolved U (DU) in Lake Biwa was reproduced by the following model and parameter research. The introduced models are the water-DU mass balance, and the ion exchange between UO
 and H
and H on the lakeshore soil. The optimized parameters were the CEC of the lakeshore, TU as the sum of DU and AU (soil adsorbed U), kads and kdes as the first order reaction rate coefficients during rapid soil adsorption and desorption of U, respectively. Tabulated by the chemical equilibria constituting DU and analyzed the contribution of each chemical species, it is shown that the seasonal variation of DU is caused by the seasonal variation of pH. A correction to the ion-exchange equilibrium to shift to first order rate reaction only when the daily AU ratio increased above kads or decreased below kdes, improved the reproducibility of DU measurements and reproduced the delay of the DU peak from the pH peak.
on the lakeshore soil. The optimized parameters were the CEC of the lakeshore, TU as the sum of DU and AU (soil adsorbed U), kads and kdes as the first order reaction rate coefficients during rapid soil adsorption and desorption of U, respectively. Tabulated by the chemical equilibria constituting DU and analyzed the contribution of each chemical species, it is shown that the seasonal variation of DU is caused by the seasonal variation of pH. A correction to the ion-exchange equilibrium to shift to first order rate reaction only when the daily AU ratio increased above kads or decreased below kdes, improved the reproducibility of DU measurements and reproduced the delay of the DU peak from the pH peak.
Matsuda, Shohei; Yokoyama, Keiichi; Yaita, Tsuyoshi; Kobayashi, Toru; Kaneta, Yui; Simonnet, M.; Sekiguchi, Tetsuhiro; Honda, Mitsunori; Shimojo, Kojiro; Doi, Reisuke; et al.
Science Advances (Internet), 8(20), p.eabn1991_1 - eabn1991_11, 2022/05
Times Cited Count:8 Percentile:41.39(Multidisciplinary Sciences)no abstracts in English
Takino, Kazuo; Sugino, Kazuteru; Oki, Shigeo
Annals of Nuclear Energy, 162, p.108454_1 - 108454_7, 2021/11
Times Cited Count:1 Percentile:9.64(Nuclear Science & Technology) C absorber material on melt progression and chemical forms of iodine or cesium under severe accident conditions
C absorber material on melt progression and chemical forms of iodine or cesium under severe accident conditionsHidaka, Akihide
Insights Concerning the Fukushima Daiichi Nuclear Accident, Vol.4; Endeavors by Scientists, p.341 - 356, 2021/10
 -ray emitter
-ray emitter 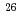 Al in massive stars; Study of the key
Al in massive stars; Study of the key 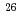 Al(n,
Al(n, ) reaction
) reactionLederer-Woods, C.*; Harada, Hideo; Kimura, Atsushi; 123 of others*
Physical Review C, 104(3), p.L032803_1 - L032803_6, 2021/09
Times Cited Count:8 Percentile:67.39(Physics, Nuclear) O
O decomposition at the U
decomposition at the U O
O surface in bicarbonate solution
surface in bicarbonate solutionMcGrady, J.; Kumagai, Yuta; Watanabe, Masayuki; Kirishima, Akira*; Akiyama, Daisuke*; Kitamura, Akira; Kimuro, Shingo
RSC Advances (Internet), 11(46), p.28940 - 28948, 2021/08
Times Cited Count:7 Percentile:24.90(Chemistry, Multidisciplinary)Harada, Masahide; Teshigawara, Makoto; Oi, Motoki; Oikawa, Kenichi; Takada, Hiroshi; Ikeda, Yujiro
Nuclear Instruments and Methods in Physics Research A, 1000, p.165252_1 - 165252_8, 2021/06
Times Cited Count:6 Percentile:43.33(Instruments & Instrumentation)This study explores high-energy neutron components of the extracted neutron beam at J-PARC pulsed neutron source using the foil activation method with threshold reactions. Foils of aluminum, gold, bismuth, niobium, and thulium were used to cover the neutron energy range from 0.3 MeV to 79.4 MeV. The experiment was performed using neutron beams of BL10 (NOBORU). The foils were irradiated by a neutron beam at 13.4 m from the moderator. To characterize high-energy neutron fields for irradiation applications, reaction rates in three different configurations with and without B C slit and Pb filter were examined. To compare the experiments with calculations given for the user, reaction rates for corresponding reactions were calculated by the PHITS code with the JENDL-3.2 and the JENDL dosimetry file. Although there was a systematic tendency in C/E (Calculation/Experiment) ratios for different threshold energies, which C/E ratio decreased as threshold energy increased up to 100 MeV, and all C/E ratios were in the range of 1.0
C slit and Pb filter were examined. To compare the experiments with calculations given for the user, reaction rates for corresponding reactions were calculated by the PHITS code with the JENDL-3.2 and the JENDL dosimetry file. Although there was a systematic tendency in C/E (Calculation/Experiment) ratios for different threshold energies, which C/E ratio decreased as threshold energy increased up to 100 MeV, and all C/E ratios were in the range of 1.0 0.2. This indicated that high-energy neutron calculations were adequate for the analysis of experimental data for NOBORU users.
0.2. This indicated that high-energy neutron calculations were adequate for the analysis of experimental data for NOBORU users.
Nishioka, Shunichiro; Nakajima, Kunihisa; Suzuki, Eriko; Osaka, Masahiko
Journal of Nuclear Science and Technology, 56(11), p.988 - 995, 2019/11
Times Cited Count:14 Percentile:77.49(Nuclear Science & Technology)In order to contribute to improvement of Cs chemisorption model used in severe accident analysis codes, the influence of chemical factors (temperature, atmosphere, concentration of affecting chemical elements etc.) on the Cs chemisorption behaviour onto stainless steel was investigated experimentally. It was found that the surface reaction rate constant used in the current Cs-chemisorption model was influenced by not only temperature, as already known, but also atmosphere, cesium hydroxide (CsOH) concentration in the gas phase and silicon content in SS304. Such chemical factors should be considered for the construction of the improved Cs-chemisorption model. Another important finding is that the chemisorption behavior at lower temperatures, around 873 K, could differ from those above 1073 K. Namely, Cs-Fe-O compounds would form as the main Cs-chemisorbed compounds at 873 K while Cs-Si-Fe-O compounds at more than 1073 K.
Miyahara, Naoya; Miwa, Shuhei; Horiguchi, Naoki; Sato, Isamu*; Osaka, Masahiko
Journal of Nuclear Science and Technology, 56(2), p.228 - 240, 2019/02
Times Cited Count:9 Percentile:62.77(Nuclear Science & Technology)In order to improve LWR source term under severe accident conditions, the first version of a fission product (FP) chemistry database named "ECUME" was developed. The ECUME is intended to include major chemical reactions and their effective kinetic constants for representative SA sequences. It is expected that the ECUME can serve as a fundamental basis from which FP chemical models in the SA analysis codes can be elaborated. The implemented chemical reactions in the first version were those for representative gas species in Cs-I-B-Mo-O-H system. The chemical reaction kinetic constants were evaluated from either literature data or calculated values using ab-initio calculations. The sample chemical reaction calculation using the presently constructed dataset showed meaningful kinetics effects at 1000 K. Comparison of the chemical equilibrium compositions by using the dataset with those by chemical equilibrium calculations has shown rather good consistency for the representative Cs-I-B-Mo-O-H species. From these results, it was concluded that the present dataset should be useful to evaluate FP chemistry in Cs-I-B-Mo-O-H system under LWA SA conditions.
Matsuda, Norihiro; Izumi, Yuichi*; Yamanaka, Yoshiyuki*; Gando, Toshiyuki*; Yamada, Masaaki*; Oishi, Koji*
EPJ Web of Conferences, 153, p.07001_1 - 07001_6, 2017/09
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Iwamoto, Hiroki; Nishihara, Kenji; Iwamoto, Yosuke; Hashimoto, Shintaro; Matsuda, Norihiro; Sato, Tatsuhiko; Harada, Masahide; Maekawa, Fujio
Journal of Nuclear Science and Technology, 53(10), p.1585 - 1594, 2016/10
Times Cited Count:19 Percentile:83.51(Nuclear Science & Technology)Kasugai, Yoshimi; Harada, Masahide; Kai, Tetsuya; Oi, Motoki; Meigo, Shinichiro; Maekawa, Fujio
JAEA-Data/Code 2015-033, 28 Pages, 2016/03
The high-energy neutron fluxes and spectra around the mercury spallation neutron source at MLF of J-PARC were measured by the multi-foil activation method. The threshold energies of neutron reactions utilized in this experiment covered from 0.1 to 50 MeV. The foil irradiation was carried out on the first beam-run of MLF from May 30th to 31th, 2008. After the irradiation, the induced radioactivity of each foil was measured using an HPGe detector, and the neutron-induced reaction-rate distribution around the mercury target was determined. Using these data, the high-energy neutron fluxes and spectra were deduced with unfolding method in which the neutron spectra calculated with PHITS code were used as the initial-guess spectra. By comparison between the initial and the unfolded spectra, it was shown that most of the calculation results, which had been the basis of the neutronics design of the MLF target assembly, were consistent with the experimental data within  30%.
30%.
Iwai, Yasunori
Fusion Engineering and Design, 98-99, p.1796 - 1799, 2015/10
Times Cited Count:5 Percentile:36.21(Nuclear Science & Technology)Hydrophobic platinum catalysts have been widely applied in the field of nuclear fusion for the exchange reactions of hydrogen isotopes between hydrogen and vapor in the water detritiation system, and for the oxidation of tritium on the atmospheric detritiation system. Hydrophobic platinum catalysts are hardly susceptible to water mist and water vapor. Hydrophobic platinum catalysts are produced by supporting platinum directly on hydrophobic polymer beads. For the hydrophobic polymer, styrene - divinyl benzene (SDB) has been applied in Japan. It can be pointed out that the upgrade in catalytic activity of hydrophobic catalyst is expected to downsize the catalytic reactor based on a hard look at a large increase in flow rate in future. The upgrade in catalytic activity of two types of commercial Pt/SDB catalysts was found when they were irradiated with electron beams. After irradiation with electron beams, the catalytic activity was evaluated by means of overall reaction rate constant for the oxidation of tritium. The overall reaction rate constant increased as increase in dose. The constant showed the peak value in the dose between 500 to 1000 kGy. After the peak, the constant decreased as increase in dose. The overall reaction rate constant at the peak was 6 times larger than that evaluated with unirradiated. The mechanical strength of irradiated Pt/SDB kept sound until 1500 kGy. The irradiation is a promising method to the upgrading in catalytic activity of Pt/SDB catalyst.
 C absorber material on melt progression and chemical forms of iodine or cesium under severe accident conditions
C absorber material on melt progression and chemical forms of iodine or cesium under severe accident conditionsHidaka, Akihide
Nihon Genshiryoku Gakkai Wabun Rombunshi, 14(1), p.51 - 61, 2015/03
B C used mainly for BWR and EPR absorbers could cause phenomena which never happen in PWR with Ag-In-Cd absorbers during severe accident. B
C used mainly for BWR and EPR absorbers could cause phenomena which never happen in PWR with Ag-In-Cd absorbers during severe accident. B C would make a eutectic interaction with stainless steel and enhance melt relocation. Boron oxidation could increase H
C would make a eutectic interaction with stainless steel and enhance melt relocation. Boron oxidation could increase H generation and change of liberated carbon to CH
generation and change of liberated carbon to CH could enhance CH
could enhance CH I generation. HBO
I generation. HBO generated during B
generated during B C oxidation could be changed to CsBO
C oxidation could be changed to CsBO by combining with Cs. This may increase Cs deposition in reactor coolant system. There could be differences in configuration, surface area, stainless steel-B
by combining with Cs. This may increase Cs deposition in reactor coolant system. There could be differences in configuration, surface area, stainless steel-B C weight ratio between B
C weight ratio between B C powder and pellet absorbers. Present issue is to clarify effect of these differences on full scale melt progression, B
C powder and pellet absorbers. Present issue is to clarify effect of these differences on full scale melt progression, B C oxidation and source term. Advancement of this research domain could contribute to further sophistication of prediction tool for melt progression and source terms, and treatment of organic iodide formation in safety evaluation.
C oxidation and source term. Advancement of this research domain could contribute to further sophistication of prediction tool for melt progression and source terms, and treatment of organic iodide formation in safety evaluation.
Hakoda, Teruyuki; Kojima, Takuji
Radiation Physics and Chemistry, 74(5), p.302 - 309, 2005/12
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)no abstracts in English
Verzilov, Y. M.; Ochiai, Kentaro; Nishitani, Takeo
Fusion Science and Technology, 48(1), p.650 - 653, 2005/07
Times Cited Count:7 Percentile:43.83(Nuclear Science & Technology)no abstracts in English
 system
systemKurosaki, Yuzuru; Takayanagi, Toshiyuki*
Chemical Physics Letters, 406(1-3), p.121 - 125, 2005/04
no abstracts in English