Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Hideya*
Journal of Nuclear Science and Technology, 61(7), p.883 - 893, 2024/07
Times Cited Count:3 Percentile:62.75(Nuclear Science & Technology)The mutual separation of Am and Cm is conducted using an alkyl-diamide amine (ADAAM) extractant. ADAAM exhibits extremely high separation factor with respect to Am and Cm separation (5.9) in a nitric acid- -dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
-dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
Micheau, C.; Ueda, Yuki; Motokawa, Ryuhei; Akutsu, Kazuhiro*; Yamada, Norifumi*; Yamada, Masako*; Moussaoui, S. A.*; Makombe, E.*; Meyer, D.*; Berthon, L.*; et al.
Journal of Molecular Liquids, 401, p.124372_1 - 124372_12, 2024/05
Times Cited Count:2 Percentile:65.57(Chemistry, Physical)Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:3 Percentile:38.46(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)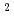
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Arai, Yoichi; Watanabe, So; Hasegawa, Kenta; Okamura, Nobuo; Watanabe, Masayuki; Takeda, Keisuke*; Fukumoto, Hiroki*; Ago, Tomohiro*; Hagura, Naoto*; Tsukahara, Takehiko*
Nuclear Instruments and Methods in Physics Research B, 542, p.206 - 213, 2023/09
Times Cited Count:1 Percentile:26.66(Instruments & Instrumentation)Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Shiwaku, Hideaki; Yaita, Tsuyoshi
RSC Advances (Internet), 13(25), p.17001 - 17007, 2023/06
Times Cited Count:3 Percentile:34.10(Chemistry, Multidisciplinary)no abstracts in English
Simonnet, M.; Sasaki, Yuji; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 41(7), p.857 - 867, 2023/00
Times Cited Count:4 Percentile:43.72(Chemistry, Multidisciplinary)Simonnet, M.; Sittel, T.*; We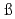 ling, P.*; Geist, A.*
ling, P.*; Geist, A.*
Energies (Internet), 15(20), p.7724_1 - 7724_10, 2022/10
Times Cited Count:5 Percentile:19.99(Energy & Fuels)Matsutani, Takafumi; Sasaki, Yuji; Katsuta, Shoichi*
Analytical Sciences, 37(11), p.1603 - 1609, 2021/11
Times Cited Count:7 Percentile:38.51(Chemistry, Analytical)We investigated the chemical behavior of lanthanides (Ln) using diglycolamide extractant with multistage extraction. We obtained the breakthrough curves for light and middle Ln. Our study reveals that the metal extraction limit depends on their  values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO
values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO , organic phase: 0.1 M TDDGA (
, organic phase: 0.1 M TDDGA (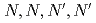 -tetradecyl-diglycolamide) in
-tetradecyl-diglycolamide) in  -dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.
-dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.
Simonnet, M.; Kobayashi, Toru; Shimojo, Kojiro; Yokoyama, Keiichi; Yaita, Tsuyoshi
Inorganic Chemistry, 60(17), p.13409 - 13418, 2021/09
Times Cited Count:25 Percentile:91.54(Chemistry, Inorganic & Nuclear)Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
JOM, 73(4), p.1037 - 1043, 2021/04
Times Cited Count:4 Percentile:29.79(Materials Science, Multidisciplinary)The separation of Dy from Nd is studied from the viewpoint of recycling Dy from Nd magnets. Both metals are lanthanide elements, which means their mutual separation is difficult because of their similar chemical behaviors. All lanthanide elements can be extracted easily by using tetradodecyl-diglycolamide (TDdDGA) extractants, and it has a relatively high separation factor (SF) between Dy and Nd (SF over 10). In the present study, by performing eight extraction steps with the organic phase (0.1M TDdDGA in dodecane), ten steps with an aqueous phase (0.7 M HNO with metals), and six steps with another aqueous phase (0.7 M HNO
with metals), and six steps with another aqueous phase (0.7 M HNO without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
Sasaki, Yuji; Morita, Keisuke; Kitatsuji, Yoshihiro; Ito, Keisuke*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 28(2), p.121 - 131, 2021/00
Times Cited Count:2 Percentile:9.00(Chemistry, Multidisciplinary)High concentration of Cs is present in high-level radioactive waste. It is well-known that Cs is an alkali element and difficult to extract completely into an organic phase. Crown ether compounds are widely available for Cs extractants; DtBuDB18C6 (di-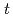 -butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as
-butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as  -dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios (
-dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios ( (Cs)), closely to 10.
(Cs)), closely to 10.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0501, p.24 - 25, 2020/12
This report summarizes the results obtained in FY2019 at Electron Linac Facility of University of Tokyo. The radiolysis process of a diglycolamide extractant, which is expected to be used in the separation process of minor actinides (MA), in dodecane and octanol solutions was investigated by pulse radiolysis. As a result, it was suggested that by adding alcohol, the decomposition process of the diglycolamide extractant was different from the decomposition processes in the single solvent of dodecane considered that the decomposition occurred via a radical cation species of the extractant.
Sasaki, Yuji; Nakase, Masahiko*
Petorotekku, 43(11), p.782 - 787, 2020/11
As analog compounds of DGA (diglycolamide), MIDOA(methylimino-diacetamide) and TDGA(thia-diglycolammide) are used for the extractants of platinum group metals. These extractants can be extracted noble metals and oxyanions, which followed by HSAB theory. The high concentration of these metals can be also extracted by these compounds. The research of metal-complex structures gives the information on the ability and role for complex-formation, which will be useful for the development of novel extractants.
 -dodecane by methylimino-dioctylacetamide (MIDOA))
-dodecane by methylimino-dioctylacetamide (MIDOA))Sasaki, Yuji
Separation Science and Technology, 55(4), p.708 - 715, 2020/00
Times Cited Count:5 Percentile:20.06(Chemistry, Multidisciplinary)Methylimino-di-n-octylacetamide (MIDOA) was used to extract 57 metals from HCl into  -dodecane. MIDOA is a tridentate ligand with one N donor and two carbonyl O donors and can extract soft acid metals and metal oxonium anions. In the present work, Fe(III), Zr(IV), Ga(III), Mo(VI), Tc(VII), Pd(II), Cd(II), In(III), Sn(IV), W(VI), Re(VII), Os(III), Ir(III), Pt(IV), Au(III), Hg(II), Bi(III), and U(VI) had distribution ratios above 10 when using 0.1 M MIDOA/n-dodecane and 3 M HCl. The amount of metal ion (Mo(VI), Tc(VII), Re(VII), Pd(II), Pt(IV), and Au(III)) extracted from HCl was greater than that obtained using HNO
-dodecane. MIDOA is a tridentate ligand with one N donor and two carbonyl O donors and can extract soft acid metals and metal oxonium anions. In the present work, Fe(III), Zr(IV), Ga(III), Mo(VI), Tc(VII), Pd(II), Cd(II), In(III), Sn(IV), W(VI), Re(VII), Os(III), Ir(III), Pt(IV), Au(III), Hg(II), Bi(III), and U(VI) had distribution ratios above 10 when using 0.1 M MIDOA/n-dodecane and 3 M HCl. The amount of metal ion (Mo(VI), Tc(VII), Re(VII), Pd(II), Pt(IV), and Au(III)) extracted from HCl was greater than that obtained using HNO .
.
Simonnet, M.; Suzuki, Shinichi; Miyazaki, Yuji*; Kobayashi, Toru; Yokoyama, Keiichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 38(4), p.430 - 440, 2020/00
Times Cited Count:23 Percentile:68.65(Chemistry, Multidisciplinary)Simonnet, M.; Miyazaki, Yuji*; Suzuki, Shinichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 37(1), p.81 - 95, 2019/00
Times Cited Count:9 Percentile:28.64(Chemistry, Multidisciplinary)Nagano, Tetsushi; Naganawa, Hirochika; Suzuki, Hideya; Toshimitsu, Masaaki*; Mitamura, Hisayoshi*; Yanase, Nobuyuki*; Grambow, B.
Analytical Sciences, 34(9), p.1099 - 1102, 2018/09
Times Cited Count:13 Percentile:43.38(Chemistry, Analytical)A previously reported emulsion flow (EF) extraction system does not include a device for refining used solvent. Therefore, the processing of large quantities of wastewater by using the EF extractor alone could lead to the accumulation of wastewater components into the solvent and diminished extraction performance. In the present study, we have developed a solvent-washing-type EF system, which is equipped with a unit for washing used solvent to prevent accumulation, and successfully applied it for treating uranium-containing wastewater.
Do, V. K.; Yamamoto, Masahiko; Taguchi, Shigeo; Kuno, Takehiko; Surugaya, Naoki
Current Analytical Chemistry, 14(2), p.111 - 119, 2018/00
Times Cited Count:4 Percentile:12.83(Chemistry, Analytical)A direct coupling of two-phase flow solvent extraction microfluidics with ICP-MS for element-selective analysis is successfully established. Two-phase flow in microchannels of two combined glass chips for continuous extraction and back-extraction is stabilized through balancing the pressure by using an external coiled tube that functions as a flow resistor. The difference of fluid flow rate between microchannels and ICP-MS is adjusted by a proposed interface system including T-junction mixer and a switching valve. An online measurement of rhenium is successfully demonstrated. The calibration curve for Re is carried out in the range of 1  g/L to 20
g/L to 20  g/L. The limit of detection is 0.2
g/L. The limit of detection is 0.2  g/L with a needed sample volume of one milliliter. Total time including extraction, back-extraction, and measurement is less than one hour. The development of the online coupling is a first step towards future applications to the selective measurement of highly radioactive elements.
g/L with a needed sample volume of one milliliter. Total time including extraction, back-extraction, and measurement is less than one hour. The development of the online coupling is a first step towards future applications to the selective measurement of highly radioactive elements.
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo; Yoshizuka, Kazuharu*
Hydrometallurgy, 169, p.576 - 584, 2017/05
Times Cited Count:16 Percentile:55.57(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (TDGA), is analogous structure to tetraoctyl-diglycolamide (TODGA) and methylimino-dioctylacetamide (MIDOA), but with sulfur donor instead of ether oxygen or nitrogen atoms of TODGA or MIDOA. From the present work, TDGA can extract silver, palladium, gold, and mercury from acidic solutions to n-dodecane. In addition to these results, the distribution ratios of hard and soft acid metals by using TDGA, TODGA, and MIDOA are compared, where the metal-complexations with each donor atom are investigated. 1H-NMR and IR studies for the metal-TDGA complexes indicate the role on donor atoms, S and N, of TDGA.
Sasaki, Yuji; Yoshimitsu, Ryo*; Nishihama, Shohei*; Shimbori, Yuma*; Shiroishi, Hidenobu*
Separation Science and Technology, 52(7), p.1186 - 1192, 2017/03
Times Cited Count:2 Percentile:8.19(Chemistry, Multidisciplinary)The new extractant, biuret(C8), is synthesized and tested for the solvent extraction of hard acid metals like actinides and soft acid metals. This compound has the similar central frame to malonamide but with 2-NH introduced into the central frame, then both amidic oxygen and nitrogen atoms may bond with metals. From the present work, not only hard acid metals, but also soft acid metals can be extracted by biuret(C8) from nitric or perchloric acids to n-dodecane. The extractability for biuret(C8) is compared with other representative extractants, malonamide, TODGA and MIDOA. It is clear that the distribution ratio(D) of U and Pu by biuret(C8) is similar to those for malonamide, but with lower values than those for TODGA and MIDOA, and D for soft acid metals and oxonium anions show higher values than those for TODGA and malonamide and lower than those for MIDOA.