Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Hideya*
Journal of Nuclear Science and Technology, 61(7), p.883 - 893, 2024/07
Times Cited Count:4 Percentile:59.85(Nuclear Science & Technology)The mutual separation of Am and Cm is conducted using an alkyl-diamide amine (ADAAM) extractant. ADAAM exhibits extremely high separation factor with respect to Am and Cm separation (5.9) in a nitric acid- -dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
-dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
Yoshida, Hidekazu*; Yamamoto, Koshi*; Asahara, Yoshihiro*; Maruyama, Ippei*; Karukaya, Koichi*; Saito, Akane*; Matsui, Hiroya; Mochizuki, Akihito; Jo, Mayumi*; Katsuta, Nagayoshi*; et al.
Communications Engineering (Internet), 3, p.67_1 - 67_10, 2024/05
A capability to permanently seal fluid flow-paths through bedrock, like boreholes or underground tunnels, is needed to ensure the long-term safety and effectiveness of many underground activities e.g. CO storage, hydrocarbon field abandonment, and nuclear waste disposal. Commonly used cementitious seals may not be sufficiently durable due to chemical and physical degradation. Learning from natural calcite (CaCO
storage, hydrocarbon field abandonment, and nuclear waste disposal. Commonly used cementitious seals may not be sufficiently durable due to chemical and physical degradation. Learning from natural calcite (CaCO ) concretion formation, a more durable sealing method was developed using a "concretion-forming solvent". The method was tested by sealing flow-paths next to a tunnel in an underground research laboratory at 350 meters depth. The flow-paths initially sealed rapidly, then resealed after disturbance by earthquakes (M5.4). The treated rock recovered its very low natural permeability, demonstrating permanent sealing that is robust.
) concretion formation, a more durable sealing method was developed using a "concretion-forming solvent". The method was tested by sealing flow-paths next to a tunnel in an underground research laboratory at 350 meters depth. The flow-paths initially sealed rapidly, then resealed after disturbance by earthquakes (M5.4). The treated rock recovered its very low natural permeability, demonstrating permanent sealing that is robust.
Micheau, C.; Ueda, Yuki; Motokawa, Ryuhei; Akutsu, Kazuhiro*; Yamada, Norifumi*; Yamada, Masako*; Moussaoui, S. A.*; Makombe, E.*; Meyer, D.*; Berthon, L.*; et al.
Journal of Molecular Liquids, 401, p.124372_1 - 124372_12, 2024/05
Times Cited Count:2 Percentile:60.60(Chemistry, Physical) evaluation tests in an Underground Research Laboratory, Horonobe, Japan
evaluation tests in an Underground Research Laboratory, Horonobe, JapanYoshida, Hidekazu*; Yamamoto, Koshi*; Asahara, Yoshihiro*; Maruyama, Ippei*; Karukaya, Koichi*; Saito, Akane*; Matsui, Hiroya; Mochizuki, Akihito; Katsuta, Nagayoshi*; Metcalfe, R.*
Powering the Energy Transition through Subsurface Collaboration; Proceedings of the 1st Energy Geoscience Conference (Energy Geoscience Conference Series, 1), 20 Pages, 2024/00
A capability to permanently seal fluid flow-paths in bedrock, such as natural faults/fractures, and damaged zones around boreholes/excavations, is needed to ensure the long-term safety and effectiveness of many underground activities. Cementitious materials are commonly used as seals, however these materials unavoidably undergo physical and chemical degradation, therefore potentially decreasing seal durability. In order to solve these problems, a more durable sealing method using concretion-forming resin has been developed by learning from natural calcite (CaCO ) concretion formation. The sealing capability of resin was tested by
) concretion formation. The sealing capability of resin was tested by  experiments on bedrock flow-paths in an underground research laboratory (URL), Hokkaido, Japan. The results showed a decrease the permeability rapidly down to 1/1,000 of the initial permeability due to calcite precipitation over a period of one year. During the experiment inland earthquakes occurred with foci below the URL (depths 2-7 km and maximum magnitude 5.4). Due to the earthquakes the hydraulic conductivities of the flow-paths sealed initially by concretion-forming resin increased. However, these flow-paths subsequently resealed rapidly, and within a few months recovered the same hydraulic conductivities as before the earthquakes. This new technique for rapidly producing long-lasting seals against fluid flow through rocks will be applicable to many kinds of underground activities.
experiments on bedrock flow-paths in an underground research laboratory (URL), Hokkaido, Japan. The results showed a decrease the permeability rapidly down to 1/1,000 of the initial permeability due to calcite precipitation over a period of one year. During the experiment inland earthquakes occurred with foci below the URL (depths 2-7 km and maximum magnitude 5.4). Due to the earthquakes the hydraulic conductivities of the flow-paths sealed initially by concretion-forming resin increased. However, these flow-paths subsequently resealed rapidly, and within a few months recovered the same hydraulic conductivities as before the earthquakes. This new technique for rapidly producing long-lasting seals against fluid flow through rocks will be applicable to many kinds of underground activities.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:3 Percentile:35.90(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Arai, Yoichi; Watanabe, So; Hasegawa, Kenta; Okamura, Nobuo; Watanabe, Masayuki; Takeda, Keisuke*; Fukumoto, Hiroki*; Ago, Tomohiro*; Hagura, Naoto*; Tsukahara, Takehiko*
Nuclear Instruments and Methods in Physics Research B, 542, p.206 - 213, 2023/09
Times Cited Count:1 Percentile:25.07(Instruments & Instrumentation)Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Shiwaku, Hideaki; Yaita, Tsuyoshi
RSC Advances (Internet), 13(25), p.17001 - 17007, 2023/06
Times Cited Count:3 Percentile:32.03(Chemistry, Multidisciplinary)no abstracts in English
Simonnet, M.; Sasaki, Yuji; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 41(7), p.857 - 867, 2023/00
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)Simonnet, M.; Sittel, T.*; We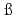 ling, P.*; Geist, A.*
ling, P.*; Geist, A.*
Energies (Internet), 15(20), p.7724_1 - 7724_10, 2022/10
Times Cited Count:5 Percentile:24.53(Energy & Fuels)Matsutani, Takafumi; Sasaki, Yuji; Katsuta, Shoichi*
Analytical Sciences, 37(11), p.1603 - 1609, 2021/11
Times Cited Count:7 Percentile:37.70(Chemistry, Analytical)We investigated the chemical behavior of lanthanides (Ln) using diglycolamide extractant with multistage extraction. We obtained the breakthrough curves for light and middle Ln. Our study reveals that the metal extraction limit depends on their  values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO
values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO , organic phase: 0.1 M TDDGA (
, organic phase: 0.1 M TDDGA ( -tetradecyl-diglycolamide) in
-tetradecyl-diglycolamide) in  -dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.
-dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.
Simonnet, M.; Kobayashi, Toru; Shimojo, Kojiro; Yokoyama, Keiichi; Yaita, Tsuyoshi
Inorganic Chemistry, 60(17), p.13409 - 13418, 2021/09
Times Cited Count:25 Percentile:91.11(Chemistry, Inorganic & Nuclear)Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
JOM, 73(4), p.1037 - 1043, 2021/04
Times Cited Count:4 Percentile:28.73(Materials Science, Multidisciplinary)The separation of Dy from Nd is studied from the viewpoint of recycling Dy from Nd magnets. Both metals are lanthanide elements, which means their mutual separation is difficult because of their similar chemical behaviors. All lanthanide elements can be extracted easily by using tetradodecyl-diglycolamide (TDdDGA) extractants, and it has a relatively high separation factor (SF) between Dy and Nd (SF over 10). In the present study, by performing eight extraction steps with the organic phase (0.1M TDdDGA in dodecane), ten steps with an aqueous phase (0.7 M HNO with metals), and six steps with another aqueous phase (0.7 M HNO
with metals), and six steps with another aqueous phase (0.7 M HNO without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
 -octylnitrilo-triacetamide (HONTA) complexes of americium and europium
-octylnitrilo-triacetamide (HONTA) complexes of americium and europiumToigawa, Tomohiro; Peterman, D. R.*; Meeker, D. S.*; Grimes, T. S.*; Zalupski, P. R.*; Mezyk, S. P.*; Cook, A. R.*; Yamashita, Shinichi*; Kumagai, Yuta; Matsumura, Tatsuro; et al.
Physical Chemistry Chemical Physics, 23(2), p.1343 - 1351, 2021/01
Times Cited Count:21 Percentile:83.18(Chemistry, Physical)The candidate An(III)/Ln(III) separation ligand hexa- -octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent (
-octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent ( -dodecane) radical cation of
-dodecane) radical cation of 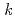 (HONTA + R
(HONTA + R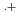 ) = (7.61
) = (7.61  0.82)
0.82)  10
10 M
M s
s obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed
obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed  -dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived (
-dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived ( 100 ns) HONTA radical cation as well as a longer-lived (
100 ns) HONTA radical cation as well as a longer-lived ( s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
Sasaki, Yuji; Morita, Keisuke; Kitatsuji, Yoshihiro; Ito, Keisuke*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 28(2), p.121 - 131, 2021/00
Times Cited Count:2 Percentile:8.76(Chemistry, Multidisciplinary)High concentration of Cs is present in high-level radioactive waste. It is well-known that Cs is an alkali element and difficult to extract completely into an organic phase. Crown ether compounds are widely available for Cs extractants; DtBuDB18C6 (di- -butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as
-butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as  -dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios (
-dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios ( (Cs)), closely to 10.
(Cs)), closely to 10.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0501, p.24 - 25, 2020/12
This report summarizes the results obtained in FY2019 at Electron Linac Facility of University of Tokyo. The radiolysis process of a diglycolamide extractant, which is expected to be used in the separation process of minor actinides (MA), in dodecane and octanol solutions was investigated by pulse radiolysis. As a result, it was suggested that by adding alcohol, the decomposition process of the diglycolamide extractant was different from the decomposition processes in the single solvent of dodecane considered that the decomposition occurred via a radical cation species of the extractant.
Sasaki, Yuji; Nakase, Masahiko*
Petorotekku, 43(11), p.782 - 787, 2020/11
As analog compounds of DGA (diglycolamide), MIDOA(methylimino-diacetamide) and TDGA(thia-diglycolammide) are used for the extractants of platinum group metals. These extractants can be extracted noble metals and oxyanions, which followed by HSAB theory. The high concentration of these metals can be also extracted by these compounds. The research of metal-complex structures gives the information on the ability and role for complex-formation, which will be useful for the development of novel extractants.
Arai, Yoichi; Watanabe, So; Ono, Shimpei; Nomura, Kazunori; Nakamura, Fumiya*; Arai, Tsuyoshi*; Seko, Noriaki*; Hoshina, Hiroyuki*; Hagura, Naoto*; Kubota, Toshio*
Nuclear Instruments and Methods in Physics Research B, 477, p.54 - 59, 2020/08
Times Cited Count:7 Percentile:55.09(Instruments & Instrumentation)Arai, Yoichi; Watanabe, So; Ono, Shimpei; Nomura, Kazunori; Nakamura, Fumiya*; Arai, Tsuyoshi*; Seko, Noriaki*; Hoshina, Hiroyuki*; Kubota, Toshio*
QST-M-23; QST Takasaki Annual Report 2018, P. 59, 2020/03
 -dodecane by methylimino-dioctylacetamide (MIDOA))
-dodecane by methylimino-dioctylacetamide (MIDOA))Sasaki, Yuji
Separation Science and Technology, 55(4), p.708 - 715, 2020/00
Times Cited Count:5 Percentile:19.73(Chemistry, Multidisciplinary)Methylimino-di-n-octylacetamide (MIDOA) was used to extract 57 metals from HCl into  -dodecane. MIDOA is a tridentate ligand with one N donor and two carbonyl O donors and can extract soft acid metals and metal oxonium anions. In the present work, Fe(III), Zr(IV), Ga(III), Mo(VI), Tc(VII), Pd(II), Cd(II), In(III), Sn(IV), W(VI), Re(VII), Os(III), Ir(III), Pt(IV), Au(III), Hg(II), Bi(III), and U(VI) had distribution ratios above 10 when using 0.1 M MIDOA/n-dodecane and 3 M HCl. The amount of metal ion (Mo(VI), Tc(VII), Re(VII), Pd(II), Pt(IV), and Au(III)) extracted from HCl was greater than that obtained using HNO
-dodecane. MIDOA is a tridentate ligand with one N donor and two carbonyl O donors and can extract soft acid metals and metal oxonium anions. In the present work, Fe(III), Zr(IV), Ga(III), Mo(VI), Tc(VII), Pd(II), Cd(II), In(III), Sn(IV), W(VI), Re(VII), Os(III), Ir(III), Pt(IV), Au(III), Hg(II), Bi(III), and U(VI) had distribution ratios above 10 when using 0.1 M MIDOA/n-dodecane and 3 M HCl. The amount of metal ion (Mo(VI), Tc(VII), Re(VII), Pd(II), Pt(IV), and Au(III)) extracted from HCl was greater than that obtained using HNO .
.
Simonnet, M.; Suzuki, Shinichi; Miyazaki, Yuji*; Kobayashi, Toru; Yokoyama, Keiichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 38(4), p.430 - 440, 2020/00
Times Cited Count:23 Percentile:68.08(Chemistry, Multidisciplinary)