Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Takahatake, Yoko; Shibata, Atsuhiro; Nomura, Kazunori; Sato, Tsutomu*
Minerals (Internet), 7(12), p.247_1 - 247_13, 2017/12
Times Cited Count:9 Percentile:41.90(Geochemistry & Geophysics)Hydrous sodium titanate (SrTreat) is able to remove radioactive Sr from Radioactive contaminated water at Fukushima Daiichi Nuclear Power station (F1NPS). Knowing the amount of radioactive nuclides in the used SrTreat is important for an effective disposal and deposition of the F1NPS waste. This study investigated changes in the ability of SrTreat to sorb Sr during its use, and to understand the causes of changes in the sorbing. After exposure to a simulated treated water for 99 h, the surface structure of the SrTreat was changed, and the percentage of sorbed Sr and the buffer capacity for protons decreased. When the amount of radioactive nuclides contained in the used SrTreat is calculated from the sorption data of the as received SrTreat.
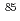 Sr,
Sr,  Np,
Np,  Pu and
Pu and  Am in loess media (Joint research)
Am in loess media (Joint research)Tanaka, Tadao; Mukai, Masayuki; Maeda, Toshikatsu; Matsumoto, Junko; Ogawa, Hiromichi; Li, S.*; Wang, Z.*; Wang, J.*; Guo, Z.*; Zhao, Y.*
JAERI-Research 2002-034, 20 Pages, 2002/12
Adsorption mechanisms and models of 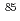 Sr(II),
Sr(II),  Np(V),
Np(V),  Pu(IV) and
Pu(IV) and  Am(III) on the loess were investigated from their adsorption and desorption properties. The distribution coefficient of
Am(III) on the loess were investigated from their adsorption and desorption properties. The distribution coefficient of 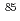 Sr and
Sr and  Np was 2 - 3 orders of magnitude smaller than that of
Np was 2 - 3 orders of magnitude smaller than that of  Pu and
Pu and  Am. The adsorption of
Am. The adsorption of 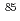 Sr and
Sr and  Np was mainly controlled by the ion exchange reaction. On the other hand, the adsorption of
Np was mainly controlled by the ion exchange reaction. On the other hand, the adsorption of  Pu and
Pu and  Am was mostly controlled by the selective chemical reactions with Fe and Mn oxyhydroxide/oxide and humic substances. On the basis of the experimental results, several types of adsorption models of the radionuclides, considering elemental concentrations, adsorption mechanisms and kinetics, were proposed for setting up the analytical systems of radionuclide migration in the loess media.
Am was mostly controlled by the selective chemical reactions with Fe and Mn oxyhydroxide/oxide and humic substances. On the basis of the experimental results, several types of adsorption models of the radionuclides, considering elemental concentrations, adsorption mechanisms and kinetics, were proposed for setting up the analytical systems of radionuclide migration in the loess media.
 -irradiated at low temperature
-irradiated at low temperatureKawatsura, Kiyoshi; Ozawa, K.; ; ;
Radiat.Eff., 22(4), p.267 - 275, 1974/04
no abstracts in English
Tachi, Yukio; Yotsuji, Kenji; Ito, Tsuyoshi; Suyama, Tadahiro
no journal, ,
The integrated sorption and diffusion (ISD) model was applied for systems coexisting multispecies Sr (divalent cation Sr and neutral SrSO
and neutral SrSO (aq)) in compacted montmorillonite. Effective diffusion coefficients (De) and distribution coefficients (Kd) of Sr in compacted Na-montmorillonite (dry density of 800 kg/m
(aq)) in compacted montmorillonite. Effective diffusion coefficients (De) and distribution coefficients (Kd) of Sr in compacted Na-montmorillonite (dry density of 800 kg/m ) saturated with three types of Na
) saturated with three types of Na SO
SO solutions (0.05, 0.1, 0.5 M) were measured by the trough-diffusion method. The De and Kd values decreased drastically with increasing porewater salinity. The De for multispecies Sr was determined as the harmonic weight-average considering the two species distribution and their log De values, based on comparison with reactive-transport calculations using the PHREEQC. As a result, the De trend could be quantitatively express by the ISD model considering multispecies contributions. The thermodynamic sorption model considering ion exchange reactions could provide reasonable account of Kd trend as functions of salinity.
solutions (0.05, 0.1, 0.5 M) were measured by the trough-diffusion method. The De and Kd values decreased drastically with increasing porewater salinity. The De for multispecies Sr was determined as the harmonic weight-average considering the two species distribution and their log De values, based on comparison with reactive-transport calculations using the PHREEQC. As a result, the De trend could be quantitatively express by the ISD model considering multispecies contributions. The thermodynamic sorption model considering ion exchange reactions could provide reasonable account of Kd trend as functions of salinity.