Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Arai, Yoichi; Watanabe, So; Hasegawa, Kenta; Okamura, Nobuo; Watanabe, Masayuki; Takeda, Keisuke*; Fukumoto, Hiroki*; Ago, Tomohiro*; Hagura, Naoto*; Tsukahara, Takehiko*
Nuclear Instruments and Methods in Physics Research B, 542, p.206 - 213, 2023/09
Times Cited Count:1 Percentile:25.07(Instruments & Instrumentation) aqueous solution system revealed by fluorescence microspectroscopy
aqueous solution system revealed by fluorescence microspectroscopyMiyagawa, Akihisa*; Kusano, Yuka*; Nagatomo, Shigenori*; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 38(7), p.955 - 961, 2022/07
Times Cited Count:1 Percentile:5.53(Chemistry, Analytical)In this study, we reveal an Eu(III) extraction mechanism at the interface between HNO and tributyl phosphate (TBP) solutions using fluorescence microspectroscopy. The mass transfer rate constant at the interface is obtained from the analysis of fluorescence intensity changes during the forward and backward extractions at various HNO
and tributyl phosphate (TBP) solutions using fluorescence microspectroscopy. The mass transfer rate constant at the interface is obtained from the analysis of fluorescence intensity changes during the forward and backward extractions at various HNO and TBP concentrations to investigate the reaction mechanism. This result indicates that one nitrate ion reacts with Eu(III) at the interface, whereas TBP molecules are not involved in the interfacial reaction, which is different from the results obtained using the NaNO
and TBP concentrations to investigate the reaction mechanism. This result indicates that one nitrate ion reacts with Eu(III) at the interface, whereas TBP molecules are not involved in the interfacial reaction, which is different from the results obtained using the NaNO solution in our previous study. We demonstrate that the chemical species of Eu(III) complex with nitrate ion and TBP in the aqueous solution play an important role for the extraction mechanism.
solution in our previous study. We demonstrate that the chemical species of Eu(III) complex with nitrate ion and TBP in the aqueous solution play an important role for the extraction mechanism.
Sano, Yuichi; Sakamoto, Atsushi; Miyazaki, Yasunori; Watanabe, So; Morita, Keisuke; Emori, Tatsuya; Ban, Yasutoshi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; et al.
Proceedings of International Conference on Nuclear Fuel Cycle; Sustainable Energy Beyond the Pandemic (GLOBAL 2022) (Internet), 4 Pages, 2022/07
We developed a hybrid MA(III) recovery process combining MA(III)+Ln(III) co-recovery flowsheet by solvent extraction with TBP and MA(III)/Ln(III) separation flowsheet by simulated moving bed chromatography using HONTA impregnated adsorbents with large particle size porous silica support.
Kusaka, Ryoji; Watanabe, Masayuki
Physical Chemistry Chemical Physics, 20(47), p.29588 - 29590, 2018/12
Times Cited Count:20 Percentile:67.81(Chemistry, Physical)Mechanistic understanding of solvent extraction of uranyl ions (UO
 ) by tributyl phosphate (TBP) will help improve the technology for the treatment and disposal of spent nuclear fuels. So far, it has been believed that uranyl ions in the aqueous phase are adsorbed to a TBP-enriched organic/aqueous interface, form complexes with TBP at the interface, and are extracted into the organic phase. Here we show that uranyl-TBP complex formation does not take place at the interface using vibrational sum frequency generation (VSFG) spectroscopy and propose an alternative extraction mechanism that uranyl nitrate, UO
) by tributyl phosphate (TBP) will help improve the technology for the treatment and disposal of spent nuclear fuels. So far, it has been believed that uranyl ions in the aqueous phase are adsorbed to a TBP-enriched organic/aqueous interface, form complexes with TBP at the interface, and are extracted into the organic phase. Here we show that uranyl-TBP complex formation does not take place at the interface using vibrational sum frequency generation (VSFG) spectroscopy and propose an alternative extraction mechanism that uranyl nitrate, UO (NO
(NO )
) , passes through the interface and forms the uranyl-TBP complex, UO
, passes through the interface and forms the uranyl-TBP complex, UO (NO
(NO )
) (TBP)
(TBP) , in the organic phase.
, in the organic phase.
Amano, Yuki; Watanabe, Koji; Masaki, Tomoo; Tashiro, Shinsuke; Abe, Hitoshi
JAEA-Technology 2016-012, 21 Pages, 2016/06
To contribute to safety evaluation of fire accident in fuel reprocessing plants, solvent extraction behavior of ruthenium, which could form volatile species, was investigated. Distribution ratios of ruthenium at fire accident conditions were obtained by extraction experiments with several solvent composition at different temperature as parameters. In order to investigate release behavior of ruthenium and europium at fire accident, release ratios of ruthenium and europium were also obtained by solvent combustion experiments.
Asakura, Toshihide; Hotoku, Shinobu; Ban, Yasutoshi; Matsumura, Masakazu; Morita, Yasuji
Journal of Nuclear and Radiochemical Sciences, 6(3), p.271 - 274, 2005/12
Tc extraction and separation experiments were performed basing on PUREX technique with using spent UO fuel with burn-up of 44 GWd/t. The experimental results were examined with performing calculations by a simulation code ESSCAR (Extraction System Simulation Code for Advanced Reprocessing). It was demonstrated that Tc can be almost quantitatively extracted from a dissolver solution and that Tc can also be almost quantitatively recovered by scrubbing. Further, it was clearly presented from the calculation results of ESSCAR that the extraction mechanism of Tc is dominated by the synergistic effect of Zr and U.
fuel with burn-up of 44 GWd/t. The experimental results were examined with performing calculations by a simulation code ESSCAR (Extraction System Simulation Code for Advanced Reprocessing). It was demonstrated that Tc can be almost quantitatively extracted from a dissolver solution and that Tc can also be almost quantitatively recovered by scrubbing. Further, it was clearly presented from the calculation results of ESSCAR that the extraction mechanism of Tc is dominated by the synergistic effect of Zr and U.
Sugikawa, Susumu; Umeda, Miki; Kobayashi, Fuyumi; Nagata, Masanobu*; Dojiri, Shigeru; Amano, Masae*
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 4 Pages, 2005/10
The mineralization of radioactive contaminated organic wastes by mediated electrochemical oxidation process has some attractive features as alternative to incineration process: The process operates safely at low temperatures and ambient pressures. JAERI has been investigated the process since 1996 and confirmed complete mineralization of this organic solvent. In order to greatly improve current efficiency for the oxidation reaction, further experiments were performed under condition of strong mixing of organic solvent and anolyte with an aide of ultrasonic wave. The current efficiencies for the oxidation reaction by ultrasonic agitation between organic solvent and anolyte were twice to that by mechanical agitation. On the basis of these results, two processes, one for destruction of a small amount of TBP/dodecane and the other for destruction of intermediate compounds following alkaline hydrolysis of a large amount of TBP/dodecane, were proposed.
Suzuki, Shinichi; Yaita, Tsuyoshi; Okamoto, Yoshihiro; Shiwaku, Hideaki; Motohashi, Haruhiko*
Physica Scripta, T115, p.306 - 307, 2005/00
In spent fuel reprocessing, distribution behavior of technetium in tri- butyl phosphate (TBP) extraction system is a very complicated. Especially, in the presence of metallic ions, i.e., U(VI), Pu(IV)and Zr(IV)in the TBP extraction system, the extraction of technetium is enhanced by the co- extraction with U, Pu and Zr. The mechanism of co-extraction mechanism of technetium is performed by exchange with nitric acid ion and per-technetate ion. In this research, we perform the structural analysis of technetium complexes in ethanol solution compared with rhenium and manganese complexes by EXAFS. Furthermore we measured some organic complexes and co-extracted complexes of technetium with uranium and zirconium extracted by TBP as compared with those of rhenium.
 -butyraldehyde in tributyl phosphate diluted with
-butyraldehyde in tributyl phosphate diluted with  -dodecane
-dodecaneBan, Yasutoshi; Asakura, Toshihide; Morita, Yasuji
Radiochimica Acta, 92(12), p.883 - 887, 2004/12
Times Cited Count:13 Percentile:62.66(Chemistry, Inorganic & Nuclear)The reduction kinetics of Np(VI) by  -butyraldehyde in 30%TBP solution diluted with
-butyraldehyde in 30%TBP solution diluted with  -dodecane were analyzed by spectrophotometry. Based on the results of both
-dodecane were analyzed by spectrophotometry. Based on the results of both  -butyraldehyde and nitric acid concentration dependencies on the Np(VI) reduction reaction, the rate equation was obtained as -d
-butyraldehyde and nitric acid concentration dependencies on the Np(VI) reduction reaction, the rate equation was obtained as -d [Np(VI)]/d
[Np(VI)]/d =
= 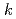 [
[ -C
-C H
H CHO]
CHO]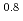 [HNO
[HNO ]
]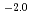 [Np(VI)]
[Np(VI)] where
where 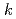 = (1.0
= (1.0 0.2)
0.2)  10
10 M
M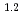 min
min at 294
at 294  1 K. The activation energy of the reaction was 76
1 K. The activation energy of the reaction was 76  5 kJ/mol. Redox equilibrium between Np(V) and N(VI), and reaction mechanism were discussed.
5 kJ/mol. Redox equilibrium between Np(V) and N(VI), and reaction mechanism were discussed.
Meguro, Yoshihiro; Tomioka, Osamu; Imai, Tomoki*; Fujimoto, Shigeyuki*; Nakashima, Mikio; Yoshida, Zenko; Honda, Tadashi*; Koya, Fumio*; Kitamura, Nobu*; Wada, Ryutaro*; et al.
Proceedings of International Waste Management Symposium 2004 (WM '04) (CD-ROM), 8 Pages, 2004/03
Supercritical CO fluid leaching (SFL) method using supercritical CO
fluid leaching (SFL) method using supercritical CO fluid containing a complex of HNO
fluid containing a complex of HNO - tri-n-butyl phosphate (TBP) was applied to removal of uranium from radioactive solid wastes. Sea sands, incineration ashes and porous alumina bricks were employed as matrixes of simulated solid wastes. Real radioactive incineration ash wastes and firebrick wastes were also subjected to the SFL treatment. It was found that uranium could be efficiently removed from both of the simulated wastes and real wastes by the SFL method. The removal efficiency of uranium from the real waste was lower than that from the corresponding artificial waste. About 1 g and 35 mg of uranium were recovered from 10 g of the real ash waste and 37 g of the real firebrick waste, respectively.
- tri-n-butyl phosphate (TBP) was applied to removal of uranium from radioactive solid wastes. Sea sands, incineration ashes and porous alumina bricks were employed as matrixes of simulated solid wastes. Real radioactive incineration ash wastes and firebrick wastes were also subjected to the SFL treatment. It was found that uranium could be efficiently removed from both of the simulated wastes and real wastes by the SFL method. The removal efficiency of uranium from the real waste was lower than that from the corresponding artificial waste. About 1 g and 35 mg of uranium were recovered from 10 g of the real ash waste and 37 g of the real firebrick waste, respectively.
Nagase, Yoshiyuki*; Masuda, Kaoru*; Wada, Ryutaro*; Yamamoto, Ichiro*; Tomioka, Osamu; Meguro, Yoshihiro; Fukuzato, Ryuichi*
Proceedings of 2nd International Symposium on Supercritical Fluid Technology for Energy and Environment Applications (Super Green 2003), p.254 - 257, 2004/00
no abstracts in English
Zhang, P.*; Kimura, Takaumi; Yoshida, Zenko
Solvent Extraction and Ion Exchange, 22(6), p.933 - 945, 2004/00
Times Cited Count:14 Percentile:46.44(Chemistry, Multidisciplinary)no abstracts in English
Matsumoto, Shiro*; Uchiyama, Gunzo; Ozawa, Masaki*; Kobayashi, Y.*; Shirato, K.*
Radiochemistry, 45(3), p.219 - 224, 2003/05
no abstracts in English
Mizumaki, Masaichiro*; Yoshii, Kenji; Kitazawa, Hideaki*; Tanida, Hajime*
Journal of Solid State Chemistry, 171(1-2), p.291 - 294, 2003/02
Times Cited Count:5 Percentile:17.13(Chemistry, Inorganic & Nuclear)no abstracts in English
 -diketone and tributylphosphate
-diketone and tributylphosphateMeguro, Yoshihiro; Iso, Shuichi; Ogiyanagi, Jin; Yoshida, Zenko
Analytical Sciences (CD-ROM), 17(Suppl.), p.721 - 724, 2002/03
no abstracts in English
 /supercritical CO
/supercritical CO +TBP system
+TBP systemMeguro, Yoshihiro; Iso, Shuichi; Yoshida, Zenko
Proceedings of International Solvent Extraction Conference 2002 (CD-ROM), p.1131 - 1136, 2002/00
no abstracts in English
 fluid leaching (SFL) of uranium from solid wastes using HNO
fluid leaching (SFL) of uranium from solid wastes using HNO -TBP complex as a reactant
-TBP complex as a reactantTomioka, Osamu*; Meguro, Yoshihiro; Iso, Shuichi; Yoshida, Zenko; Enokida, Yoichi*; Yamamoto, Ichiro*
Proceedings of International Solvent Extraction Conference 2002 (CD-ROM), p.1143 - 1147, 2002/00
no abstracts in English
Clifford, A. A.*; Zhu, S.*; Smart, N. G.*; Lin, Y.*; Wai, C. M.*; Yoshida, Zenko; Meguro, Yoshihiro; Iso, Shuichi
Journal of Nuclear Science and Technology, 38(6), p.433 - 438, 2001/06
Times Cited Count:14 Percentile:68.29(Nuclear Science & Technology)no abstracts in English
 medium containing HNO
medium containing HNO -TBP complex
-TBP complexTomioka, Osamu*; Meguro, Yoshihiro; Iso, Shuichi; Yoshida, Zenko; Enokida, Yoichi*; Yamamoto, Ichiro*
Journal of Nuclear Science and Technology, 38(6), p.461 - 462, 2001/06
Times Cited Count:27 Percentile:85.12(Nuclear Science & Technology)no abstracts in English
Takanashi, Mitsuhiro; ; Aoshima, Atsushi
JNC TN8400 2001-022, 60 Pages, 2001/03
A numerical simulation code for the TRUEX (Transuranium Extraction) process was developed. Concentration profiles of americium and europium were calculated for some experiments of the counter current extraction system those were carried out in CPF (Chemical Processing Facility) by using the code. Calculation profiles were in agreement with the experimental results. Operational conditions were also examinted for the americium recovery experiment by the TRUEX process carried out in the Plutonium Fuel Center. It was shown that lowering the concentration of nitric acid in the scrub solution and decreasing the flow rate of solvent and strip solution was effective for improving the performance of the stripping step and reducing the volume of the waste solution. In order to find the optimum conditions for various experiments, this simulation code was modified to calculate the concentration profiles of other metal elements such as zirconium and iron and the effect of oxalic acid on the extraction behavior of the metal elements. The calculated concentration profiles of americium and europium were varied by this modification. In the experiment at CPF, the calculations were carried out to obtain recovery ratio of americium in the product stream with the amount of oxalic acid added to the process. This calculation result showed that it was possible to improve the performance of decontamination of fission products by increasing oxalic acid concentration added to the process. The calculation was also carried out for finding the optimum conditions of oxalic acid concentration added to the europium recovery process.