Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Tatsuya*
Solvent Extraction Research and Development, Japan, 32(1), p.21 - 29, 2025/00
The magnitude of the masking effect of carboxylic, amic-acidic, and amidic compounds through Ln and Am extractions by tetraoctyl diglycolamide (TODGA) is compared. The compounds used are diglycol, ethylenediamine, diethylenetriamine-type, and two other amides (dioxaoctane diamide and nitrylotriacetamide). The results show that below pH 1.2, where carboxylic acids are less dissociated, amide O atoms have higher reactivity with lanthanides than O atoms in carboxyl groups. From observing the Ln patterns (D(Ln) vs. their atomic number), the compounds primarily show high reactivity, with middle and heavy Ln having a higher charge density than light Ln. Four amide compounds are employed in this work. Those with tertiary amine N atoms have pH dependence on D(Ln) due to protonation and dissociation from amine N atoms.
 -tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degree
-tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degreeMiyagawa, Akihisa*; Takahashi, Takumi*; Kuzure, Yoshiaki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 39(11), p.1929 - 1936, 2023/11
Times Cited Count:1 Percentile:10.71(Chemistry, Analytical)In this study, we revealed the Eu(III) distribution in a single diglycolamide-derivative extractant (TODGA)-impregnated polymer-coated silica particle. The reaction of Eu(III) with two TODGA molecules in the polymer layer was the rate-limiting process, as evidenced by the absence of any correlation between the rate constants (k and k
and k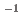 ) and concentrations of Eu(III) and HNO
) and concentrations of Eu(III) and HNO .
.
Nomizu, Daiki; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Katsuta, Shoichi*
Journal of Radioanalytical and Nuclear Chemistry, 331(3), p.1483 - 1493, 2022/03
Times Cited Count:7 Percentile:73.20(Chemistry, Analytical)We studied the successive formation of water soluble DGA (diglycolamide) and DOODA (dioxaoctanediamide) for the mutual separation of Ln in this extraction system. TODGA (tetraoctyl-diglycolamide) and DOODA(C8) (tetraoctyl-dioxaoctanediamide) have the opposite trend to extract light and heavy Ln through Ln-patterns. Metal-complexes of two folding Ln ions with water-soluble DOODA and three folding with DGA are found and their observed formation constants are calculated. The suitable separation condition (aqueous phase: 30 mM DOODA(C2) in 1 M nitric acid, organic phase: 0.1 M TODGA in n-dodecane) of multi-stage extraction (10  10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:5 Percentile:28.02(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Solvent Extraction and Ion Exchange, 40(6), p.620 - 640, 2022/00
Times Cited Count:2 Percentile:12.88(Chemistry, Multidisciplinary)Owing to the chemical behavior of trivalent lanthanide and actinide ions with similar ionic radii, realizing this separation is still challenging. All lanthanides, Am, and Cm can be extracted using diglycolamide (DGA), and relatively high An/Ln separation efficiencies have been obtained using diethylenetriamine-triacetic-bisamide (DTBA). To improve the previous results as well as the separation conditions, we used organic acids for pH adjustment. The advantages of this modification included low HNO , DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
, DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
Matsumiya, Masahiko*; Tsuchida, Yusuke*; Sasaki, Yuji; Ono, Ryoma*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Radioanalytical and Nuclear Chemistry, 327(1), p.597 - 607, 2021/01
Times Cited Count:4 Percentile:34.76(Chemistry, Analytical)To achieve trichotomic separation of light lanthanides (Ln), heavy Ln, and Am, batchwise multi-stage extractions using tetraoctyl-diglycolamide (TODGA) extractant from organic acids are studied. Malonic acid (MA) has high solubility in water and is used as the main component of the aqueous phase. It is clear that the separation factor (SF) for Nd/Am from MA and that for La/Am from MA + HNO are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO
are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO (1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
(1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
Toigawa, Tomohiro; Tsubata, Yasuhiro; Kai, Takeshi; Furuta, Takuya; Kumagai, Yuta; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 39(1), p.74 - 89, 2021/00
Times Cited Count:2 Percentile:7.36(Chemistry, Multidisciplinary)Absorbed-dose estimation is essential for evaluation of the radiation feasibility of minor-actinide-separation processes. We propose a dose-evaluation method based on radiation permeability, with comparisons of heterogeneous structures seen in the solvent-extraction process, such as emulsions forming in the mixture of the organic and aqueous phases. A demonstration of radiation-energy-transfer simulation is performed with a focus on the minor-actinide-recovery process from high-level liquid waste with the aid of the Monte Carlo radiation-transport code PHITS. The simulation results indicate that the dose absorbed by the extraction solvent from alpha ray depends upon the emulsion structure, and that from beta and gamma ray depends upon the mixer-settler-apparatus size. Non-negligible contributions of well-permeable gamma rays were indicated in terms of the plant operation of the minor-actinide-separation process.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0501, p.24 - 25, 2020/12
This report summarizes the results obtained in FY2019 at Electron Linac Facility of University of Tokyo. The radiolysis process of a diglycolamide extractant, which is expected to be used in the separation process of minor actinides (MA), in dodecane and octanol solutions was investigated by pulse radiolysis. As a result, it was suggested that by adding alcohol, the decomposition process of the diglycolamide extractant was different from the decomposition processes in the single solvent of dodecane considered that the decomposition occurred via a radical cation species of the extractant.
 -tetraoctyl-diglycolamide from organic acid
-tetraoctyl-diglycolamide from organic acidSasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 49(10), p.1216 - 1219, 2020/10
Times Cited Count:9 Percentile:35.36(Chemistry, Multidisciplinary)Lanthanide (Ln) extractions from organic acids to  -dodecane by
-dodecane by  -tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio (
-tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio ( ) greater 1 was obtained for heavy Ln. Ln patterns (
) greater 1 was obtained for heavy Ln. Ln patterns ( (Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high
(Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high  values, the addition of HNO
values, the addition of HNO in aqueous phase is found to be effective.
in aqueous phase is found to be effective.
 system
systemSasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Radiochimica Acta, 108(9), p.689 - 699, 2020/09
Times Cited Count:10 Percentile:64.46(Chemistry, Inorganic & Nuclear)The simultaneous separation of Am and Cm from lanthanides is important for atomic energy fields. All lanthanides, Am, and Cm can be extracted by diglycolamide (DGA). In addition, relatively high separation factors between An and Ln were obtained by the extraction system of TODGA, DTPA (diethylenetriamine-pentaacetic acid) and HNO . In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
. In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
Sasaki, Yuji; Saeki, Morihisa*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 26(1), p.21 - 34, 2019/06
Times Cited Count:6 Percentile:21.71(Chemistry, Multidisciplinary)Three tridentate extractants and three masking reagents including O, N, and S donors have been developed and their properties are compared and discussed. The extractants are termed as tetraoctyl-diglycolamide (TODGA), methylimino-dioctylacetamide (MIDOA) and tetraoctyl-thiodiglycolamide (TDGA(C8)) and masking agents have the same central frame but with short alkyl chain. The results of the present study indicate that TODGA can extract mainly hard acid metals belonging groups 2-4,13-15 in periodic table, MIDOA can extract soft acid metals and oxyanions (groups 5-10, 16), and TDGA can extract soft acid metals (groups 10-11). Some spectrophotometric studies (UV, IR, and NMR) indicate the stoichiometry and effect of donor atoms for metal-complexation. The Hf values, the heat generation during complex formation, obtained by chemical calculation by DFT theory show the reverse-correlation with their extraction ability.
Sasaki, Yuji; Morita, Keisuke
Progress in Nuclear Science and Technology (Internet), 5, p.27 - 32, 2018/12
no abstracts in English
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo*; Yoshizuka, Kazuharu*
Proceedings of 21st International Solvent Extraction Conference (ISEC 2017) (Internet), p.131 - 134, 2017/11
Three tridentate extractants including soft and hard donor has been developed and examined. The extractants are termed as 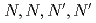 -tetraoctyl-diglycolamide (TODGA), methylimino-
-tetraoctyl-diglycolamide (TODGA), methylimino-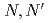 -dioctylacetamide (MIDOA) and
-dioctylacetamide (MIDOA) and 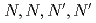 -tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
-tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
Sasaki, Yuji; Morita, Keisuke; Ito, Keisuke; Suzuki, Shinichi; Shiwaku, Hideaki; Takahashi, Yuya*; Kaneko, Masaaki*; Omori, Takashi*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference (GLOBAL 2017) (USB Flash Drive), 4 Pages, 2017/09
no abstracts in English
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo; Yoshizuka, Kazuharu*
Hydrometallurgy, 169, p.576 - 584, 2017/05
Times Cited Count:16 Percentile:55.22(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (TDGA), is analogous structure to tetraoctyl-diglycolamide (TODGA) and methylimino-dioctylacetamide (MIDOA), but with sulfur donor instead of ether oxygen or nitrogen atoms of TODGA or MIDOA. From the present work, TDGA can extract silver, palladium, gold, and mercury from acidic solutions to n-dodecane. In addition to these results, the distribution ratios of hard and soft acid metals by using TDGA, TODGA, and MIDOA are compared, where the metal-complexations with each donor atom are investigated. 1H-NMR and IR studies for the metal-TDGA complexes indicate the role on donor atoms, S and N, of TDGA.
Sasaki, Yuji; Yoshimitsu, Ryo*; Nishihama, Shohei*; Shimbori, Yuma*; Shiroishi, Hidenobu*
Separation Science and Technology, 52(7), p.1186 - 1192, 2017/03
Times Cited Count:2 Percentile:8.14(Chemistry, Multidisciplinary)The new extractant, biuret(C8), is synthesized and tested for the solvent extraction of hard acid metals like actinides and soft acid metals. This compound has the similar central frame to malonamide but with 2-NH introduced into the central frame, then both amidic oxygen and nitrogen atoms may bond with metals. From the present work, not only hard acid metals, but also soft acid metals can be extracted by biuret(C8) from nitric or perchloric acids to n-dodecane. The extractability for biuret(C8) is compared with other representative extractants, malonamide, TODGA and MIDOA. It is clear that the distribution ratio(D) of U and Pu by biuret(C8) is similar to those for malonamide, but with lower values than those for TODGA and MIDOA, and D for soft acid metals and oxonium anions show higher values than those for TODGA and malonamide and lower than those for MIDOA.
Nagoshi, Kohei*; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Sato, Mutsumi*; Oikawa, Hiroshi*
Nihon Ion Kokan Gakkai-Shi, 28(1), p.11 - 18, 2017/01
no abstracts in English
Watanabe, So; Sano, Yuichi; Kofuji, Hirohide; Takeuchi, Masayuki; Koizumi, Tsutomu
NEA/NSC/R(2015)2 (Internet), p.338 - 344, 2015/06
 -dodecane containing a solvent modifier
-dodecane containing a solvent modifierSasaki, Yuji; Zhu, Z.-X.; Sugo, Yumi; Suzuki, Hideya*; Kimura, Takaumi
Analytical Sciences, 21(10), p.1171 - 1175, 2005/10
Times Cited Count:30 Percentile:62.22(Chemistry, Analytical)In order to evaluate the extraction property of the new extractants, diglycolamide(DGA) compounds, we investigated the maximum extraction of di-, tri-, and tetravalent metal ions using the system of nitric acid and n-dodecane. Limits of metal concentration (LOC) for Ca(II), Nd(III) and Zr(IV) influence strongly on HNO and the extractant concentration. On the purpose to raise the LOC value, we employed the modifier of solvent, N,N-dihexyl-octanamide(DHOA) and DGA with the long alkyl chain, and examined. It is evident that LOC increases with DHOA concentration in n-dodecane and the length of alkyl chain attached with N atom of DGA. The stoichiometric values of LOC(Zr) estimated from the extraction reaction is obtained by using the extraction condition: tetraoctyl-DGA/ 1M DHOA+n-dodecane and 3M HNO
and the extractant concentration. On the purpose to raise the LOC value, we employed the modifier of solvent, N,N-dihexyl-octanamide(DHOA) and DGA with the long alkyl chain, and examined. It is evident that LOC increases with DHOA concentration in n-dodecane and the length of alkyl chain attached with N atom of DGA. The stoichiometric values of LOC(Zr) estimated from the extraction reaction is obtained by using the extraction condition: tetraoctyl-DGA/ 1M DHOA+n-dodecane and 3M HNO .
.
Sasaki, Yuji; Zhu, Z.-X.; Sugo, Yumi; Kimura, Takaumi
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 5 Pages, 2005/10
The extraction of metals by N,N,N',N'-tetraoctyl-diglycolamide (TODGA) from nitric acid to n-dodecane was investigated. From the measurement of distribution ratios, it was obvious that Ca (ionic radius: 100pm), trivalent and tetravalent ions with ionic radii of 87-113 and 83-94 pm are highly extractable by TODGA. In order to evaluate the extraction capacity in extraction solvent using DGA compounds, we measured the limits of metal concentration (LOC) for Ca(II), Nd(III) and Zr(IV). For the purpose to enhance the LOC value, we examined the modifier of solvent, N,N-dihexyl-octanamide(DHOA) and DGA with longer alkyl chain. It is evident that LOC is increased with DHOA concentration and the length of alkyl chain attached to N atom of DGA.