Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kato, Hiroshige*; Sato, Mitsuyoshi*; Owada, Hitoshi*; Mihara, Morihiro;
JNC TN8430 2000-008, 53 Pages, 2000/05
Cementitious materials will be used in TRU waste disposal repository. In such cases, it is considered that the migration of alkaline leachates from cementitious materials, so called high pH plume, will cause dissolution of rock and precipitation of secondary minerals. In addition, the high pH plume will move along the flow of groundwater, so it is predicted that rock formation and components of high pH groundwater vary with time and space. However, time and spatial dependence of the variations of secondary minerals and groundwater components has not been clarified. In order to acquire the data of variations of secondary minerals and groundwater components, we carried out the rock alteration experiments with column method. The crushed granodiorite was filled into 4 meters length column (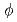 3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80
3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80 C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
; Nishikawa, Yoshiaki*; Kagawa, Akio;
JNC TN8400 2000-017, 30 Pages, 2000/03
The influence of the cement additives on the distribution coefficients of americium-241 to the Ca-bentonite was confirmed. The adsorption experiment of americium-241 to Ca-bentonite with cement additives was performed by the batch method, as a part of the influence evaluation of the organic in the research of TRU waste disposal. As a result, the distribution coefficient of americium-241 to the Ca-bentonite was over 1.2E+3m /kg in the condition of the absence of cement additives. In the case of low concentration (0.3g/kg) of the naphthalenesulfonic acid type cement additives, the distribution coefficient was 5.2E+2m
/kg in the condition of the absence of cement additives. In the case of low concentration (0.3g/kg) of the naphthalenesulfonic acid type cement additives, the distribution coefficient was 5.2E+2m kg. And, in the case of high concentration (30g/kg) of the same cement additives, the distribution coefficients was 2.0E-1m
kg. And, in the case of high concentration (30g/kg) of the same cement additives, the distribution coefficients was 2.0E-1m /kg. On the other hand in the case of flow concentration (0.5g/kg) of the polycarboxylic acid type cement additives, the distribution coefficients was over 1.3E+3m
/kg. On the other hand in the case of flow concentration (0.5g/kg) of the polycarboxylic acid type cement additives, the distribution coefficients was over 1.3E+3m /kg. And, in the case of high concentration (50g/kg) of the same cement additives, the distribution coefficient was 1.8E-1m
/kg. And, in the case of high concentration (50g/kg) of the same cement additives, the distribution coefficient was 1.8E-1m /kg. Here, selected cement additives concentrations were based on a standard concentration of 10g/kg when the ratio of water:cement is about one. From these results, the distribution coefficient of americium-241 to the Ca-bentonite decreases according cement additive concentration. The distribution coefficients were similar on different kinds of cement additives. The cement additives concentration influences the distribution coefficient. The distribution coefficient was small in the case of high concentration of the cement additives. That is, it is thought that the cement additives have small influences on the distribution coefficient of americium-241 to the Ca-bentonite in the case of low concentration, though the cement additives have influences on the distribution coefficient of americium-241 to the Ca-bentonite in the case of high concentration.
/kg. Here, selected cement additives concentrations were based on a standard concentration of 10g/kg when the ratio of water:cement is about one. From these results, the distribution coefficient of americium-241 to the Ca-bentonite decreases according cement additive concentration. The distribution coefficients were similar on different kinds of cement additives. The cement additives concentration influences the distribution coefficient. The distribution coefficient was small in the case of high concentration of the cement additives. That is, it is thought that the cement additives have small influences on the distribution coefficient of americium-241 to the Ca-bentonite in the case of low concentration, though the cement additives have influences on the distribution coefficient of americium-241 to the Ca-bentonite in the case of high concentration.
 -isosaccharinic acid, 2
-isosaccharinic acid, 2Iwata, Hajime; Kimuro, Shingo; Kitamura, Akira; Miyabe, Shunsuke*; Maeno, Mamiko*; Tanaka, Takeru*; Hieda, Manami*
no journal, ,
The effect of  -isosaccharinic acid (ISA) on the solubility of palladium (Pd) in alkaline aqueous solution (pH 8.5, 10.0) was investigated by solubility experiments. The Pd concentration increased with the increase of ISA concentration. The equilibrium constant was calculated from the solubility data obtained in this study.
-isosaccharinic acid (ISA) on the solubility of palladium (Pd) in alkaline aqueous solution (pH 8.5, 10.0) was investigated by solubility experiments. The Pd concentration increased with the increase of ISA concentration. The equilibrium constant was calculated from the solubility data obtained in this study.
Kitamura, Akira; Kobayashi, Taishi*; Sasaki, Takayuki*
no journal, ,
Solubility of zirconium was investigated in highly basic solutions containing calcium to evaluate effect of cementitious materials for setting parameters on radionuclide migration for TRU waste disposal. The obtained aqueous concentration of zirconium as functions of hydrogen and calcium concentrations was compared with thermodynamic calculations using published thermodynamic data.