Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ueda, Yuki; Micheau, C.; Motokawa, Ryuhei
Fuain Kemikaru, 54(5), p.53 - 60, 2025/05
no abstracts in English
Takahashi, Yoshio*; Yamaguchi, Akiko; Yomogida, Takumi
Treatise on Geochemistry, 3rd edition, Vol.6, p.105 - 150, 2025/00
With the recent development of measurement techniques, new approaches to the environmental geochemistry of radionuclides have been applied for various research targets. In this review article, several topics within the last 10-15 years in the field of environmental geochemistry of radionuclides have been discussed. In particular, this article mainly focused on two topics, (i) studies on the migration of radionuclides emitted by the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident in 2011 and (ii) the development of X-ray absorption fine structure (XAFS) spectroscopy and its application to the geochemical processes of radionuclides.
Minowa, Kazuki*; Watanabe, So; Nakase, Masahiko*; Takahatake, Yoko; Miyazaki, Yasunori; Ban, Yasutoshi; Matsuura, Haruaki*
Nuclear Instruments and Methods in Physics Research B, 556, p.165496_1 - 165496_6, 2024/11
Times Cited Count:0 Percentile:0.00(Instruments & Instrumentation)In this study, X-ray absorption near edge structure (XANES) spectral analysis and column experiments were used to verify the selectivity of rare earth (RE) ions by alkyl diamide amine (ADAAM) adsorbent. In addition, the interactions between the N atoms of ADAAM and RE ions were evaluated to determine whether any of the RE ions are a valid simulant for developing a mutual separation process for minor actinides (MAs) in highly radioactive liquid waste. It was confirmed that La and Ce interacted with the amine N atom of ADAAM and they showed a peak shift of the N-K edge XANES spectrum; this finding suggested that a soft interaction is an essential factor influencing ion selectivity. Therefore, the selection factor of RE ions by ADAAM adsorbent was similar to that of MAs. It was concluded that RE ions are reasonable species to simulate MAs.
Kobayashi, Taishi*; Sato, Yutaro*; Tonna, Ryutaro*; Matsumura, Daiju; Sasaki, Takayuki*; Ikeda, Atsushi
Dalton Transactions (Internet), 53(46), p.18616 - 18628, 2024/10
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)Kato, Hiroyuki S.*; Muroyama, Mizuho*; Kobayakawa, Nano*; Muneyasu, Riku*; Tsuda, Yasutaka; Murase, Natsumi*; Watanabe, Seiya*; Yamada, Takashi*; Kanematsu, Yusuke*; Tachikawa, Masanori*; et al.
Journal of Physical Chemistry Letters (Internet), 15(43), p.10769 - 10776, 2024/10
Times Cited Count:1 Percentile:42.16(Chemistry, Physical)Ikeda, Atsushi
Bunseki Kagaku, 73(4.5), p.147 - 159, 2024/04
Factor analysis (FA), one of the multivariate analysis methods that are frequently employed in chemometrics, is a powerful tool to extract physical/chemical information of pure components from the analytical data of the mixture of the components. Although FA has been applied mainly to the quantitative analysis in the field of analytical chemistry, its application to the qualitative analysis has been limited. This article aims at introducing FA to the qualitative analysis for determining the speciation/structure of individual chemical species/compounds by X-ray absorption spectroscopy (XAS). The fundamental concept to extract physically/chemically meaningful information from XAS-FA is described, and some reported studies using XAS-FA for the qualitative determination of chemical species/compounds are summarized.
Yomogida, Takumi; Hashimoto, Tadashi; Okumura, Takuma*; Yamada, Shinya*; Tatsuno, Hideyuki*; Noda, Hirofumi*; Hayakawa, Ryota*; Okada, Shinji*; Takatori, Sayuri*; Isobe, Tadaaki*; et al.
Analyst, 149(10), p.2932 - 2941, 2024/03
Times Cited Count:1 Percentile:40.01(Chemistry, Analytical)In this study, we successfully applied a transition-edge sensor (TES) spectrometer as a detector for microbeam X-ray measurements from a synchrotron X-ray light source to determine uranium (U) distribution at the micro-scale and its chemical species in biotite obtained from the U mine. It is difficult to separate the fluorescent X-ray of the U L
 line at 13.615 keV from that of the Rb K
line at 13.615 keV from that of the Rb K line at 13.395 keV in the X-ray fluorescence spectrum with an energy resolution of approximately 220 eV of the conventional silicon drift detector (SDD). Meanwhile, the fluorescent X-rays of U L
line at 13.395 keV in the X-ray fluorescence spectrum with an energy resolution of approximately 220 eV of the conventional silicon drift detector (SDD). Meanwhile, the fluorescent X-rays of U L
 and Rb K
and Rb K were fully separated by TES with 50 eV energy resolution at the energy of around 13 keV. The successful peak separation by TES led to an accurate mapping analysis of trace U in micro-X-ray fluorescence measurements and a decrease in the signal-to-background ratio in micro-X-ray absorption near edge structure spectroscopy.
were fully separated by TES with 50 eV energy resolution at the energy of around 13 keV. The successful peak separation by TES led to an accurate mapping analysis of trace U in micro-X-ray fluorescence measurements and a decrease in the signal-to-background ratio in micro-X-ray absorption near edge structure spectroscopy.
 Ge
Ge
Fujiwara, Hidenori*; Nakatani, Yasuhiro*; Aratani, Hidekazu*; Kanai-Nakata, Yuina*; Yamagami, Kohei*; Hamamoto, Satoru*; Kiss, Takayuki*; Sekiyama, Akira*; Tanaka, Arata*; Ebihara, Takao*; et al.
New Physics; Sae Mulli, 73(12), p.1062 - 1066, 2023/12
 Na
Na TiO
TiO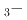 BaTiO
BaTiO solid solutions
solid solutionsYoneda, Yasuhiro; Kobayashi, Toru; Tsuji, Takuya; Matsumura, Daiju; Saito, Yuji; Noguchi, Yuji*
Japanese Journal of Applied Physics, 62(SM), p.SM1006_1 - SM1006_8, 2023/11
Times Cited Count:4 Percentile:50.15(Physics, Applied)Bi Na
Na TiO
TiO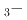 BaTiO
BaTiO (BNT
(BNT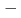 BT) solid solutions have been extensively studied because they exhibit good piezoelectric properties. In addition, a wide variety of phases are observed depending on the BT composition. Soft X-ray absorption spectroscopy and high-energy X-ray diffraction experiments of BNT
BT) solid solutions have been extensively studied because they exhibit good piezoelectric properties. In addition, a wide variety of phases are observed depending on the BT composition. Soft X-ray absorption spectroscopy and high-energy X-ray diffraction experiments of BNT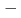 BT solid solutions were performed using synchrotron radiation. From the electronic structure and local structure of BNT
BT solid solutions were performed using synchrotron radiation. From the electronic structure and local structure of BNT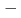 BT solid solution, the substitution effect of BT occurred mainly at the A site, which is the substitution site of Ba. The rhombohedral strain of the TiO
BT solid solution, the substitution effect of BT occurred mainly at the A site, which is the substitution site of Ba. The rhombohedral strain of the TiO octahedron did not change with the change in BT composition, suggesting that the change in the electronic structure at the O-
octahedron did not change with the change in BT composition, suggesting that the change in the electronic structure at the O- absorption edge is due to the change in the hybridization state.
absorption edge is due to the change in the hybridization state.
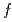 symmetry for anisotropic
symmetry for anisotropic 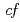 hybridization in the heavy-fermion superconductor CeNi
hybridization in the heavy-fermion superconductor CeNi Ge
Ge
Fujiwara, Hidenori*; Nakatani, Yasuhiro*; Aratani, Hidekazu*; Kanai-Nakata, Yuina*; Yamagami, Kohei*; Hamamoto, Satoru*; Kiss, Takayuki*; Yamasaki, Atsushi*; Higashiya, Atsushi*; Imada, Shin*; et al.
Physical Review B, 108(16), p.165121_1 - 165121_10, 2023/10
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Nakae, Masanori*; Matsuyama, Tsugufumi*; Murakami, Masashi; Yoshida, Yukihiko; Machida, Masahiko; Tsuji, Koichi*
Advances in X-Ray Chemical Analysis, Japan, 54, p.89 - 99, 2023/03
Fundamental research on X-ray absorption imaging for elemental identification was studied. A secondary target was applied to obtain X-ray absorption images above and below the X-ray absorption edge of the target element. X-rays from an X-ray tube were irradiated to the secondary target, where the characteristic X-rays were emitted that were irradiated to the sample. X-ray absorption images were acquired with an exposure time of a few seconds with an X-ray camera. In this technique, it is difficult to change the energy of X-rays as we want, however we can apply this technique for imaging the specific element. Metal foil sample composed of Al, Cu, and Ni was analyzed. To obtain an X-ray elemental image of Ni, two X-ray absorption images were taken using the X-rays above and below the Ni K-edge. X-rays of Cu K and Zn K
and Zn K were prepared by using Cu and Zn plates as the secondary target. Finally, the Ni elemental image was obtained by subtracting two images. Furthermore, the X-ray camera had a function of setting critical energies for imaging, thus it was demonstrated that an X-ray elemental image of Ni was obtained using a single secondary target without changing the secondary target.
were prepared by using Cu and Zn plates as the secondary target. Finally, the Ni elemental image was obtained by subtracting two images. Furthermore, the X-ray camera had a function of setting critical energies for imaging, thus it was demonstrated that an X-ray elemental image of Ni was obtained using a single secondary target without changing the secondary target.
 -edge to assess the U(V) electronic structure in FeUO
-edge to assess the U(V) electronic structure in FeUO
Yomogida, Takumi; Akiyama, Daisuke*; Ouchi, Kazuki; Kumagai, Yuta; Higashi, Kotaro*; Kitatsuji, Yoshihiro; Kirishima, Akira*; Kawamura, Naomi*; Takahashi, Yoshio*
Inorganic Chemistry, 61(50), p.20206 - 20210, 2022/12
Times Cited Count:7 Percentile:59.79(Chemistry, Inorganic & Nuclear)FeUO was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L
was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L -edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
-edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
 Sr
Sr CoO
CoO (0
(0 0.5)
0.5)Matsumura, Daiju; Tsuji, Takuya; Yoshii, Kenji
Materials Chemistry and Physics, 238, p.121885_1 - 121885_5, 2019/12
Times Cited Count:2 Percentile:6.50(Materials Science, Multidisciplinary)We have conducted X-ray absorption spectroscopy study for perovskite cobaltite Pr Sr
Sr CoO
CoO (0
(0 0.5) to understand Sr doping effect and magnetocaloric measurement for Pr
0.5) to understand Sr doping effect and magnetocaloric measurement for Pr Sr
Sr CoO
CoO to reveal the origin of magnetic anomaly at around 110 K. The extended X-ray absorption fine structure (EXAFS) measurements at the Co
to reveal the origin of magnetic anomaly at around 110 K. The extended X-ray absorption fine structure (EXAFS) measurements at the Co  -edge suggest that the average valence value of Co ions increases, the interatomic distance of Co-O bonding decreases, and the local distortion of CoO
-edge suggest that the average valence value of Co ions increases, the interatomic distance of Co-O bonding decreases, and the local distortion of CoO octahedron increases as Sr is doped in PrCoO
octahedron increases as Sr is doped in PrCoO . The interatomic distance of Co-O bonding shrinks more greatly for the larger doping region. No temperature dependent electronic and structural changes were observed from Co
. The interatomic distance of Co-O bonding shrinks more greatly for the larger doping region. No temperature dependent electronic and structural changes were observed from Co  -edge X-ray absorption spectra in the whole thermal range studied (17-300 K). The magnetocaloric effect of Pr
-edge X-ray absorption spectra in the whole thermal range studied (17-300 K). The magnetocaloric effect of Pr Sr
Sr CoO
CoO indicates the change of magnetic structure at 110 K.
indicates the change of magnetic structure at 110 K.
Wu, H.*; Wang, Y.*; Ikeda, Atsushi; Miller, C. J.*; Waite, T. D.*
Environmental Science; Water Research & Technology, 5(8), p.1400 - 1411, 2019/08
Times Cited Count:7 Percentile:31.31(Engineering, Environmental)In this study, the distributions of iron and phosphorus species in a 1.25 m pilot scale submerged membrane bioreactor dosed with Fe(II) salts to either the membrane chamber or the 1st anoxic chamber were determined using X-ray absorption spectroscopy (XAS) at the iron and phosphorus K-edges. Significant differences in the distribution of Fe species were evident at the commencement of dosing depending on the chamber to which Fe(II) was dosed though these differences were much less distinct by the time steady state conditions were achieved. Both the co-precipitation of P with Fe and adsorption of phosphorus to iron oxides play important roles with regard to the removal of phosphorus from the MBR supernatant with the results of this work suggesting that P removal via formation of Fe(III)-phosphate mineral species is preferred if Fe(II) is dosed to the membrane chamber rather than the 1st anoxic chamber.
pilot scale submerged membrane bioreactor dosed with Fe(II) salts to either the membrane chamber or the 1st anoxic chamber were determined using X-ray absorption spectroscopy (XAS) at the iron and phosphorus K-edges. Significant differences in the distribution of Fe species were evident at the commencement of dosing depending on the chamber to which Fe(II) was dosed though these differences were much less distinct by the time steady state conditions were achieved. Both the co-precipitation of P with Fe and adsorption of phosphorus to iron oxides play important roles with regard to the removal of phosphorus from the MBR supernatant with the results of this work suggesting that P removal via formation of Fe(III)-phosphate mineral species is preferred if Fe(II) is dosed to the membrane chamber rather than the 1st anoxic chamber.
Saeki, Morihisa*; Matsumura, Daiju; Yomogida, Takumi; Taguchi, Tomitsugu*; Tsuji, Takuya; Saito, Hiroyuki*; Oba, Hironori*
Journal of Physical Chemistry C, 123(1), p.817 - 824, 2019/01
Times Cited Count:17 Percentile:53.18(Chemistry, Physical)Reaction kinetics of laser-induced particle formation in an aqueous solution of PdCl
 was investigated by transmission electron microscope (TEM) and dispersive X-ray absorption fine structure (DXAFS). The Pd particle was generated by irradiation of nanosecond pulsed 266-nm laser. The TEM observation showed dependence of the particle size on the laser fluence and promotion of the particle growth by irradiation of high-fluence laser. The DXAFS data give us the Pd
was investigated by transmission electron microscope (TEM) and dispersive X-ray absorption fine structure (DXAFS). The Pd particle was generated by irradiation of nanosecond pulsed 266-nm laser. The TEM observation showed dependence of the particle size on the laser fluence and promotion of the particle growth by irradiation of high-fluence laser. The DXAFS data give us the Pd concentration. Temporal changes of the Pd
concentration. Temporal changes of the Pd concentration analyzed based on Finke-Watzky two step mechanism. The analysis elucidates that the laser photon contributes to the reduction of the PdCl
concentration analyzed based on Finke-Watzky two step mechanism. The analysis elucidates that the laser photon contributes to the reduction of the PdCl
 ion by the one-photon process and to the autocatalytic growth of the Pd particles by the multi-photon process.
ion by the one-photon process and to the autocatalytic growth of the Pd particles by the multi-photon process.
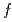 symmetry in CeCu
symmetry in CeCu Ge
Ge ; Soft X-ray absorption and hard X-ray photoemission spectroscopy
; Soft X-ray absorption and hard X-ray photoemission spectroscopyAratani, Hidekazu*; Nakatani, Yasuhiro*; Fujiwara, Hidenori*; Kawada, Moeki*; Kanai-Nakata, Yuina*; Yamagami, Kohei*; Fujioka, Shuhei*; Hamamoto, Satoru*; Kuga, Kentaro*; Kiss, Takayuki*; et al.
Physical Review B, 98(12), p.121113_1 - 121113_6, 2018/09
Times Cited Count:5 Percentile:22.43(Materials Science, Multidisciplinary)Shimoyama, Iwao; Baba, Yuji; Hirao, Norie*
Advances in Engineering (Internet), 1 Pages, 2018/02
The performance of organic devices largely depends on molecular orientation in organic films. Whereas micro-orientation control of organic molecules is an indispensable technology for integration of organic devices, the method has not been established. We attempted to control micro-orientation of polydimethylsilane (PDMS) thin films by deposition of PDMS on graphite substrates modified by hetero atom doping using ion beam. Polarization dependence measurements of X-ray absorption spectroscopy and molecular orbital calculations clarified that PDMS films have lying, standing, and random orientations on the non irradiated, N
 -irradiated, and Ar
-irradiated, and Ar -irradiated graphite surfaces, respectively. Furthermore, photoemission microscopy observation clarified that a PDMS film showed micro-patterns on a graphite surface with a microstructure on the order of
-irradiated graphite surfaces, respectively. Furthermore, photoemission microscopy observation clarified that a PDMS film showed micro-patterns on a graphite surface with a microstructure on the order of  m by separating N
m by separating N
 -irradiated and non irradiated areas. These results demonstrate our method is promising for micro-orientation of organic molecules.
-irradiated and non irradiated areas. These results demonstrate our method is promising for micro-orientation of organic molecules.
 O
O induced by swift heavy ions
induced by swift heavy ionsYoshioka, Satoru*; Tsuruta, Konosuke*; Yamamoto, Tomokazu*; Yasuda, Kazuhiro*; Matsumura, Sho*; Ishikawa, Norito; Kobayashi, Eiichi*
Physical Chemistry Chemical Physics, 20(7), p.4962 - 4969, 2018/02
Times Cited Count:7 Percentile:29.06(Chemistry, Physical)Cationic disorder in the MgAl O
O spinel induced by swift heavy ions was investigated using the X-ray absorption near edge structure. With changes in the irradiation fluences of 200 MeV Xe ions, the Mg K-edge and Al K-edge spectra were synchronously changed. The calculated spectra based on density function theory indicate that the change in the experimental spectra was due to cationic disorder between Mg in tetrahedral sites and Al in octahedral sites. These results suggest a high inversion degree to an extent that the completely random configuration is achieved in MgAl
spinel induced by swift heavy ions was investigated using the X-ray absorption near edge structure. With changes in the irradiation fluences of 200 MeV Xe ions, the Mg K-edge and Al K-edge spectra were synchronously changed. The calculated spectra based on density function theory indicate that the change in the experimental spectra was due to cationic disorder between Mg in tetrahedral sites and Al in octahedral sites. These results suggest a high inversion degree to an extent that the completely random configuration is achieved in MgAl O
O induced by the high density electronic excitation under swift heavy ion irradiation.
induced by the high density electronic excitation under swift heavy ion irradiation.
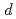 atoms in Ni
atoms in Ni Fe
Fe N (
N ( = 1 and 3) films revealed by X-ray absorption spectroscopy and magnetic circular dichroism
= 1 and 3) films revealed by X-ray absorption spectroscopy and magnetic circular dichroismTakata, Fumiya*; Ito, Keita*; Takeda, Yukiharu; Saito, Yuji; Takanashi, Koki*; Kimura, Akio*; Suemasu, Takashi*
Physical Review Materials (Internet), 2(2), p.024407_1 - 024407_5, 2018/02
Times Cited Count:15 Percentile:45.34(Materials Science, Multidisciplinary)Saeki, Morihisa*; Taguchi, Tomitsugu*; Oba, Hironori*; Matsumura, Daiju; Tsuji, Takuya; Yomogida, Takumi
Denki Gakkai Kenkyukai Shiryo, Denshi Zairyo Kenkyukai (EFM-17-010 021), p.15 - 18, 2017/09
021), p.15 - 18, 2017/09
Irradiation of nanosecond pulsed UV laser into a solution of palladium ion leads to formation of palladium particles with sub-micron size particles by time-resolved X-ray absorption spectroscopy.