Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fablet, L.*; P drot, M.*; Choueikani, F.*; Kieffer, I.*; Proux, O.*; Pierson-Wickmann, A.-C.*; Cagniart, V.*; Yomogida, Takumi; Marsac, R.*
drot, M.*; Choueikani, F.*; Kieffer, I.*; Proux, O.*; Pierson-Wickmann, A.-C.*; Cagniart, V.*; Yomogida, Takumi; Marsac, R.*
Environmental Science; Nano, 12(5), p.2815 - 2827, 2025/05
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Nickel is an omnipresent trace element in the environment. Due to its high affinity for iron oxide nanoparticles, its elimination from soils and water by these nanoparticles represents an interesting strategy, specially by magnetites, which is naturally present in the environment. However, the interactions between Ni and magnetite are poorly understood, because of the difficulty to control the stoichiometry (Fe(II)-to-Fe(III) ratio) of magnetite. The behavior of Ni in the presence of magnetite nanoparticles with different stoichiometries, in aqueous solution and inert atmosphere, are probed by adsorption experiments and X-ray Absorption Spectroscopy. This study helps predicting the interactions between Ni and magnetite in environmental conditions, which can be used for the development of efficient remediation strategies.
Ueda, Yuki; Micheau, C.; Motokawa, Ryuhei
Fuain Kemikaru, 54(5), p.53 - 60, 2025/05
no abstracts in English
 Pt
Pt Ga
Ga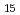 with a honeycomb structure using extended X-ray absorption fine structure
with a honeycomb structure using extended X-ray absorption fine structureMatsumoto, Yuji*; Watabe, Yuki*; Iesari, F.*; Osumi, Masakatsu*; Ota, Kyugo*; Haga, Yoshinori; Hatada, Keisuke*; Okajima, Toshihiko*
Metals, 15(4), p.436_1 - 436_13, 2025/04
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary) crystals
crystalsTakatori, Sayuri*; Pimon, M.*; Pollitt, S.*; Bartokos, M.*; Beeks, K.*; Gr neis, A.*; Hiraki, Takahiro*; Homma, Tetsuo*; Hosseini, N.*; Leitner, A.*; et al.
neis, A.*; Hiraki, Takahiro*; Homma, Tetsuo*; Hosseini, N.*; Leitner, A.*; et al.
New Journal of Physics (Internet), 27(4), p.043024_1 - 043024_10, 2025/04
Times Cited Count:0 Percentile:0.00(Physics, Multidisciplinary)Recent reports on laser excitation of the low-energy thorium-229 (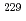 Th) nuclear isomeric state in calcium fluoride single crystals render this system a promising candidate for a solid-state nuclear clock. However, experimental characterization of the microscopic ion arrangement around the doped
Th) nuclear isomeric state in calcium fluoride single crystals render this system a promising candidate for a solid-state nuclear clock. However, experimental characterization of the microscopic ion arrangement around the doped 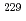 Th and its electronic charge state, crucial for the precise control of the clock transition and assessing the solid-state clock's performance, remains an unresolved task. This study uses X-ray absorption fine structure spectroscopy of
Th and its electronic charge state, crucial for the precise control of the clock transition and assessing the solid-state clock's performance, remains an unresolved task. This study uses X-ray absorption fine structure spectroscopy of 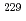 Th:CaF
Th:CaF to investigate the charge state and coordination environment of doped
to investigate the charge state and coordination environment of doped 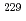 Th. The results indicate that
Th. The results indicate that 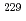 Th is doped with a 4+ valence at the substitutional site of the Ca
Th is doped with a 4+ valence at the substitutional site of the Ca ion, with charge compensated provided by two F
ion, with charge compensated provided by two F ions located at interstitial sites adjacent to
ions located at interstitial sites adjacent to 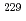 Th.
Th.
Yomogida, Takumi; Takahashi, Yoshio*
Chikyu Kagaku, 59(1), p.1 - 10, 2025/03
X-ray absorption fine structure XAFS spectroscopy techniques, which are applicable to almost all elements, provide information on elemental valence and local structure with high elemental selectivity and high sensitivity. It has become an indispensable method in space geochemistry and environmental chemistry. This review presents examples of the application of fluorescence XAFS methods to elements that are difficult to detect by conventional methods, and examples where new chemical species information has been obtained by increasing the energy resolution of the X-ray fluorescence (XRF) detection system to obtain XAFS.
Scaria, J.*; P drot, M.*; Fablet, L.*; Yomogida, Takumi; Nguyen, T. T.*; Sivry, Y.*; Catrouillet, C.*; Pradas del Real, A. E.*; Choueikani, F.*; Vantelon, D.*; et al.
drot, M.*; Fablet, L.*; Yomogida, Takumi; Nguyen, T. T.*; Sivry, Y.*; Catrouillet, C.*; Pradas del Real, A. E.*; Choueikani, F.*; Vantelon, D.*; et al.
Environmental Science & Technology, 59(11), p.5747 - 5755, 2025/03
Times Cited Count:1 Percentile:0.00(Engineering, Environmental)Understanding and predicting the interaction mechanisms between chromium and magnetite is of particular interest to elucidate the biogeochemical behavior of Cr in the environment and to develop optimal soil remediation and water treatment strategies. However, while the elimination of the most toxic form of (Cr(VI)) by its reduction to Cr(III) has widely been documented, elucidating the exact mechanism involved in Cr(III) sorption to magnetite has attracted less attention. This study examined the interaction of Cr(III) solution with 10 nm-sized magnetites, whose stoichiometries were carefully defined and preserved in anaerobic conditions. This study reveals the joint effects of pH and magnetite stoichiometry on Cr(III) sorption mechanism, and that Cr(III)-(hydr)oxide precipitation is not necessarily the driving process of Cr(III) elimination from solutions. These results will help predict the fate and transport of chromium, as well as developing magnetite-based chromium remediation processes.
 O
O , FeUO
, FeUO , and UO
, and UO -Zr-stainless steel system samples generated in an oxidative atmosphere in the presence of malonic acid
-Zr-stainless steel system samples generated in an oxidative atmosphere in the presence of malonic acidTonna, Ryutaro*; Sasaki, Takayuki*; Okamoto, Yoshihiro; Kobayashi, Taishi*
Journal of Nuclear Materials, 605, p.155561_1 - 155561_9, 2025/02
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Kato, Yuto*; Sasaki, Takayuki*; Tonna, Ryutaro*; Kobayashi, Taishi*; Okamoto, Yoshihiro
Applied Geochemistry, 175, p.106196_1 - 106196_9, 2024/11
Times Cited Count:1 Percentile:41.61(Geochemistry & Geophysics)Ueda, Yuki; Micheau, C.; Akutsu, Kazuhiro*; Tokunaga, Kohei; Yamada, Masako*; Yamada, Norifumi*; Bourgeois, D.*; Motokawa, Ryuhei
Langmuir, 40(46), p.24257 - 24271, 2024/11
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Microscopic structures in liquid-liquid extraction, such as structuration between extractants or extracted complexes in bulk organic phases and at interfaces, can influence macroscopic phenomena, such as the distribution behavior of solutes, including extraction efficiency, selectivity, and phase separation of the organic phase. In this study, we correlated the macroscopic behavior of the extraction of Zr(IV) ions from nitric acid solutions with microscopic structural information in order to understand at the molecular level the key factors contributing to the higher metal ion extraction performance in the fluorous extraction system comprising fluorous phosphate (TFP) in perfluorohexane as compared to the analogous organic extraction system comprising organic phosphate (THP) in n-hexane. Extended X-ray absorption fine structure, neutron reflectometry, and small-angle neutron scattering revealed the local coordination structure around the Zr(IV) ion, the accumulation of extractant molecules at the interface, and the structuration of extractant molecules in the bulk extraction phase, respectively. In the fluorous extraction system, extractant aggregates with were formed after contact with nitric acid. In contrast, in the organic extraction system, only extractant dimers were formed. We speculate that differences in the local coordination structure around the Zr(IV) ion and the structuration of the extractant molecules in the bulk extraction phase contribute to the high Zr(IV) extraction performance in the fluorous extraction system. In particular, the size of the aggregates hardly changed with increasing Zr(IV) concentration in the fluorous phase, which may be closely related to the absence of phase splitting in the fluorous extraction system.
Tsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 12(5), p.259 - 262, 2024/10
no abstracts in English
Krajewska, A.*; Tsuji, Takuya; Matsumura, Daiju; 15 of others*
Science Advances (Internet), 10(41), p.eadn3880_1 - eadn3880_10, 2024/10
Times Cited Count:3 Percentile:43.84(Multidisciplinary Sciences)
Yamamoto, Hajime*; Ikeda, Osamu*; Honda, Takashi*; Kimura, Kenta*; Aoyama, Takuya*; Ogushi, Kenya*; Suzuki, Akio*; Ishii, Kenji*; Matsumura, Daiju; Tsuji, Takuya; et al.
Physical Review Materials (Internet), 8(9), p.094402_1 - 094402_6, 2024/09
Times Cited Count:2 Percentile:51.02(Materials Science, Multidisciplinary)Nankawa, Takuya
Kagaku, 79(8), p.48 - 52, 2024/08
Lacquer is a natural paint with excellent water and chemical resistance. It has long been known that adding a very small amount of iron into raw lacquer produces a very beautiful black color called Shikoku. However, the structure and reactions of lacquer are still largely unknown, and the reason of black color is also unclear. In this study, we analyzed the structure of raw lacquer and black lacquer films by using different kind of quantum beams. As a result, black lacquer has a completely different nanostructure from raw lacquer, and that the color changes depending on the difference in the structure. This result is the first successful analysis of the structure of the lacquer film, which had been a mystery for many years. This commentary describes this research and also explains how this research has been proceeded.
Jung, H.*; Matsumura, Daiju; 12 of others*
ACS Energy Letters (Internet), 9(5), p.2162 - 2172, 2024/04
Times Cited Count:11 Percentile:92.35(Chemistry, Physical)Yamamoto, Yuri*; Minowa, Kazuki*; Takahatake, Yoko; Watanabe, So; Nakamura, Masahiro; Matsuura, Haruaki*
Electrochemistry (Internet), 92(4), p.043019_1 - 043019_4, 2024/04
Times Cited Count:0 Percentile:0.00(Electrochemistry)In the pyro-reprocessing of spent nuclear fuel, salt bath is normally used in several times, but at final moment, spent salt containing small amount of chloride nuclear fuel material is generated. In terms of managing nuclear fuel materials, it is desirable that the nuclear fuel materials should be recovered from the spent salt. We are proposing methods for recovering nuclear fuel materials using precipitation by oxide addition and distillation for reducing pressure techniques. In this study, we have focused on the behavior of manganese(II), which is one of the radioactivated products. As a result of experiments and thermodynamic simulation, it was found that manganese(II) is likely to be entrained in nuclear fuel materials. Therefore, it is necessary to add a step to separate manganese(II) from nuclear fuel materials.
Lei, Y.-J.*; Matsumura, Daiju; 15 of others*
Nature Communications (Internet), 15, p.3325_1 - 3325_12, 2024/04
Times Cited Count:38 Percentile:98.58(Multidisciplinary Sciences) O
O -75MoO
-75MoO binary glasses composed of isolated MoO
binary glasses composed of isolated MoO

Masuno, Atsunobu*; Munakata, Sae*; Okamoto, Yoshihiro; Yaji, Toyonari*; Kosugi, Yoshihisa*; Shimakawa, Yuichi*
Inorganic Chemistry, 63(12), p.5701 - 5708, 2024/03
Times Cited Count:2 Percentile:60.73(Chemistry, Inorganic & Nuclear)Transparent and brown La O
O -MoO
-MoO binary glasses were prepared in bulk form using a levitation technique. The glass-forming range was limited, with the primary composition being approximately 25 mol% La
binary glasses were prepared in bulk form using a levitation technique. The glass-forming range was limited, with the primary composition being approximately 25 mol% La O
O . This glass exhibited a clear crystallization at 546
. This glass exhibited a clear crystallization at 546  C, while determining its glass transition temperature was difficult. Notably, despite its amorphous nature, the glass possessed a density and packing density comparable to those of crystalline La
C, while determining its glass transition temperature was difficult. Notably, despite its amorphous nature, the glass possessed a density and packing density comparable to those of crystalline La Mo
Mo O
O . X-ray absorption fine structure and Raman scattering analyses revealed that the glass structure closely resembles La
. X-ray absorption fine structure and Raman scattering analyses revealed that the glass structure closely resembles La Mo
Mo O
O due to the presence of isolated MoO
due to the presence of isolated MoO
 units, whereas disordered atomic arrangement around La atoms was confirmed. The glass demonstrated transparency ranging from 378 to 5500 nm, and the refractive index at 1.0
units, whereas disordered atomic arrangement around La atoms was confirmed. The glass demonstrated transparency ranging from 378 to 5500 nm, and the refractive index at 1.0  m was estimated to be 2.0. The optical bandgap energy was 3.46 eV, which was slightly smaller than that of La
m was estimated to be 2.0. The optical bandgap energy was 3.46 eV, which was slightly smaller than that of La Mo
Mo O
O . Additionally, the glass displayed a transparent region ranging from 6.5 to 8.0
. Additionally, the glass displayed a transparent region ranging from 6.5 to 8.0  m. This occurrence results from the decreased diversity of MoO
m. This occurrence results from the decreased diversity of MoO units and connectivity of Mo-O-Mo, which resulted in the reduced overlap of multiphonon absorption. This glass formation, with its departure from conventional glass-forming rules, resulted in distinctive glasses with crystal-like atomic arrangements.
units and connectivity of Mo-O-Mo, which resulted in the reduced overlap of multiphonon absorption. This glass formation, with its departure from conventional glass-forming rules, resulted in distinctive glasses with crystal-like atomic arrangements.
Tonna, Ryutaro*; Sasaki, Takayuki*; Okamoto, Yoshihiro; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*
Journal of Nuclear Materials, 589, p.154862_1 - 154862_10, 2024/02
Times Cited Count:2 Percentile:43.92(Materials Science, Multidisciplinary)The dissolution behavior of FeUO compounds formed by a high-temperature reaction of UO
compounds formed by a high-temperature reaction of UO with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U
with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U O
O and Fe
and Fe O
O as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO
as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO
compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U
dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U O
O ).
).
Nagai, Takayuki; Katsuoka, Nanako; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*
Photon Factory Activity Report 2023 (Internet), 3 Pages, 2024/00
no abstracts in English
Nagai, Takayuki; Hasegawa, Takehiko*
JAEA-Research 2023-008, 41 Pages, 2023/12
To reduce the risks posed by stored the high-level radioactive liquid waste (HAW), Tokai Vitrification Facility (TVF) is working to produce the HAW into vitrified bodies. With the aim of steady vitrification of HAW in TVF, the vitrification technology section has manufactured a new 3rd melter with an improved bottom structure and is working to verify the performance of this melter. In this study, solidified glass samples were taken from simulated vitrified bodies produced by flowing molten glass during the bottom drain-out test in the operation confirmation of the TVF 3rd melter. And the properties of the surface layer and fracture surface of the vitrified bodies were evaluated by using Raman spectroscopy, synchrotron radiation XAFS measurement, and laser ablation inductively coupled plasma atomic emission spectroscopy (LA ICP-AES) analysis. As a result of measuring the surface layer and fracture surface of the solidified samples produced on an actual scale, a slight difference was confirmed between the properties of the surface layer and those of the fracture surface. Since the chemical composition of these simulated vitrified bodies does not contain platinum group elements, it is expected that the glass structure of solidified glass samples is different from that of the actual vitrified body. However, this sample measuring was a valuable opportunity to evaluate samples produced by using the direct energized joule heating method. The properties of cullet used the operation confirmation of the TVF 3rd melter and the cullet of another production lot were measured and analyzed in the same manner under the measuring conditions of solidified glass samples. As a result, it was confirmed that cullet with different producing histories have different glass structures even with the same chemical composition, and that differences in glass structures remain in the glass samples after melting these cullet.