Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -contamination visualization (Contract research); FY2022 Nuclear Energy Science & Technology and Human Resource Development Project
-contamination visualization (Contract research); FY2022 Nuclear Energy Science & Technology and Human Resource Development ProjectCollaborative Laboratories for Advanced Decommissioning Science; Hokkaido University*
JAEA-Review 2024-006, 54 Pages, 2024/06
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2022. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station (1F), Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2022, this report summarizes the research results of the "Development of elemental technologies of hand-foot-cloth monitors for  -contamination visualization" conducted in FY2022. The present study aims to develop hand-foot-monitors for
-contamination visualization" conducted in FY2022. The present study aims to develop hand-foot-monitors for  -contamination visualization and cloth monitors for
-contamination visualization and cloth monitors for  /
/ -contamination visualization consisting of a portable phoswich detector for measuring
-contamination visualization consisting of a portable phoswich detector for measuring  /
/ -contamination distribution and energy to ensure the safety and security of workers involved in the decommissioning project of the 1F. The possibility of practical application of new scintillator materials and devices was examined with the goal of developing such new instruments.
-contamination distribution and energy to ensure the safety and security of workers involved in the decommissioning project of the 1F. The possibility of practical application of new scintillator materials and devices was examined with the goal of developing such new instruments.
Yamagata, Kazuhito*; Ouchi, Kazuki; Marumo, Kazuki*; Tasaki-Handa, Yuiko*; Haraga, Tomoko; Saito, Shingo*
Inorganic Chemistry, 62(2), p.730 - 738, 2023/01
Times Cited Count:3 Percentile:81.71(Chemistry, Inorganic & Nuclear)The inert NpO
 complex with a fluorescein-modified phenanthroline-2,9-dicarboxylic acid was found by kinetic selection using polyacrylamide gel electrophoresis (PAGE) from a small chemical library. The small spontaneous dissociation rate constant of 8
complex with a fluorescein-modified phenanthroline-2,9-dicarboxylic acid was found by kinetic selection using polyacrylamide gel electrophoresis (PAGE) from a small chemical library. The small spontaneous dissociation rate constant of 8 10
10 s
s (the half-life of 23 hours) was determined. This is the singly-charged NpO
(the half-life of 23 hours) was determined. This is the singly-charged NpO
 complex exhibiting unusual kinetic inertness in aqueous solution, one million times slower than widely accepted fast kinetics of neptunyl complexes. Selective fluorescence detection of NpO
complex exhibiting unusual kinetic inertness in aqueous solution, one million times slower than widely accepted fast kinetics of neptunyl complexes. Selective fluorescence detection of NpO
 was achieved in PAGE with a detection limit of 68 pmol dm
was achieved in PAGE with a detection limit of 68 pmol dm (17 fg). This system was successfully applied to simulated spent nuclear fuel and high-level radioactive waste samples.
(17 fg). This system was successfully applied to simulated spent nuclear fuel and high-level radioactive waste samples.
Omasa, Yoshinori*; Takagi, Shigeyuki*; Toshima, Kento*; Yokoyama, Kaito*; Endo, Wataru*; Orimo, Shinichi*; Saito, Hiroyuki*; Yamada, Takeshi*; Kawakita, Yukinobu; Ikeda, Kazutaka*; et al.
Physical Review Research (Internet), 4(3), p.033215_1 - 033215_9, 2022/09
Ikeda, Takashi; Suzuki, Shinichi; Yaita, Tsuyoshi
Journal of Physical Chemistry A, 119(30), p.8369 - 8375, 2015/07
Times Cited Count:21 Percentile:65.69(Chemistry, Physical)Adsorption states of alkali metal ions in three kinds of 2:1 type clay minerals are systematically investigated via first-principles-based metadynamics. Our reconstructed free energy surfaces in a two dimensional space of coordination numbers specifically employed as collective variables for describing the interlayer cations show that an inner-sphere (IS) complex is preferentially formed for Cs in the 2:1 type trioctahedral clay minerals with saponitelike compositions, where lighter alkali ions show a tendency to form an outer-sphere one instead. The strong preference for an IS complex observed for Cs
in the 2:1 type trioctahedral clay minerals with saponitelike compositions, where lighter alkali ions show a tendency to form an outer-sphere one instead. The strong preference for an IS complex observed for Cs is found to result partially from the capability of recognizing selectively Cs
is found to result partially from the capability of recognizing selectively Cs ions at basal O atoms with the Lewis basicity significantly enhanced by the isomorphic substitution in tetrahedral sheets.
ions at basal O atoms with the Lewis basicity significantly enhanced by the isomorphic substitution in tetrahedral sheets.
 -dimethylformamide
-dimethylformamideKim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Ikeda, Yasuhisa*
Journal of Alloys and Compounds, 408-412, p.1291 - 1295, 2006/02
Times Cited Count:10 Percentile:54.49(Chemistry, Physical)The electrochemical reactions of UO (
( -diketonato)
-diketonato) DMF, UO
DMF, UO (trop)
(trop) DMF and UO
DMF and UO (sap)(DMF)
(sap)(DMF) , (DMF = N,N-dimethylformamide,
, (DMF = N,N-dimethylformamide,  -diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E
-diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E ,
, 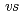 . ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO
. ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO (ttfa)
(ttfa) DMF, -1.18 V for UO
DMF, -1.18 V for UO (btfa)
(btfa) DMF, -1.46 V for UO
DMF, -1.46 V for UO (dbm)
(dbm) DMF, -1.46 V for UO
DMF, -1.46 V for UO (trop)
(trop) DMF, and -1.59 V for UO
DMF, and -1.59 V for UO (sap)(DMF)
(sap)(DMF) .
.
 emission spectroscopy of layered manganate La
emission spectroscopy of layered manganate La Sr
Sr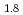 Mn
Mn O
O
Agui, Akane; K
 mbre, T.*; S
mbre, T.*; S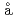 the, C.*; Nordgren, J.*; Usuda, Manabu; Saito, Tomohiko*; Moritomo, Yutaka*
the, C.*; Nordgren, J.*; Usuda, Manabu; Saito, Tomohiko*; Moritomo, Yutaka*
Journal of Electron Spectroscopy and Related Phenomena, 144-147, p.589 - 592, 2005/06
Times Cited Count:1 Percentile:5.66(Spectroscopy)Current research in the perovskite and layered perovskite-type manganese oxides has expanded to include the study of electronic and magnetic properties such as the colossal magnetoresistance (CMR), orbital ordering and charge ordering. The O K soft X-ray emission and absorption spectra (XES and XAS) of La
soft X-ray emission and absorption spectra (XES and XAS) of La Sr
Sr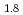 Mn
Mn O
O were measured at selected excitation energies in order to investigate the electronic structure. The measurements were carried out by using a soft X-ray emission spectrometer at beamline BW3 at HASYLAB, Germany. The dependence of O K
were measured at selected excitation energies in order to investigate the electronic structure. The measurements were carried out by using a soft X-ray emission spectrometer at beamline BW3 at HASYLAB, Germany. The dependence of O K XES on excitation-energy was observed. It is attributed the different site of oxygen ions.
XES on excitation-energy was observed. It is attributed the different site of oxygen ions.
 Emission spectra of layered manganite La
Emission spectra of layered manganite La Sr
Sr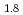 Mn
Mn O
O
Agui, Akane; Butorin, S. M.*; K
 mbre, T.*; S
mbre, T.*; S the, C.*; Saito, Tomohiko*; Moritomo, Yutaka*; Nordgren, J.*
the, C.*; Saito, Tomohiko*; Moritomo, Yutaka*; Nordgren, J.*
Journal of the Physical Society of Japan, 74(6), p.1772 - 1776, 2005/06
Times Cited Count:8 Percentile:48.22(Physics, Multidisciplinary)no abstracts in English
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Uchiyama, Gunzo*; Ikeda, Yasuhisa*
Radiochimica Acta, 93(2), p.75 - 81, 2005/02
Times Cited Count:9 Percentile:52.95(Chemistry, Inorganic & Nuclear)no abstracts in English
 (NO
(NO )
) ;M=Cs,Rb,K,NH
;M=Cs,Rb,K,NH )
);
Spectrochimica Acta, Part A, 51(3), p.309 - 318, 1995/00
no abstracts in English
A.Li*; H.Huang*; D.Li*; S.Zheng*; H.Du*; S.Zhu*; Iwata, Tadao
Japanese Journal of Applied Physics, 32(3), p.1033 - 1038, 1993/03
Times Cited Count:8 Percentile:44.65(Physics, Applied)no abstracts in English
Nagaoka, Yoshiharu; Saito, Minoru;
Transactions of the American Nuclear Society, 66, p.454 - 455, 1992/11
no abstracts in English
Iyoku, Tatsuo; Shiozawa, Shusaku; Ishihara, Masahiro; ; Oku, Tatsuo*
Nucl. Eng. Des., 132, p.23 - 30, 1991/00
Times Cited Count:23 Percentile:89.71(Nuclear Science & Technology)no abstracts in English
Radioisotopes, 35(11), p.571 - 576, 1986/11
no abstracts in English
Applied Spectroscopy, 34(3), p.327 - 331, 1980/00
no abstracts in English
Journal of Inorganic and Nuclear Chemistry, 41(8), p.1145 - 1147, 1979/00
Times Cited Count:7no abstracts in English
Spectrochimica Acta, Part A, 35A(1), p.99 - 104, 1979/00
no abstracts in English
Spectrochimica Acta, Part A, 35A(11), p.1283 - 1288, 1979/00
no abstracts in English
Journal of Inorganic and Nuclear Chemistry, 40(7), p.1369 - 1374, 1978/07
Times Cited Count:20no abstracts in English
;
Analytica Chimica Acta, 96, p.211 - 214, 1978/00
Times Cited Count:0no abstracts in English
J.Coord.Chem., 8, p.35 - 39, 1978/00
Times Cited Count:12no abstracts in English