Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
Applied Clay Science, 195, p.105741_1 - 105741_11, 2020/09
Times Cited Count:3 Percentile:11.75(Chemistry, Physical)Safety functions for the clay buffer in a repository for high-level radioactive waste (HLW) are fulfilled if the presence of montmorillonite with high swelling capacity and low permeability is maintained in the long-term. The transformation of montmorillonite to the non-swelling mineral likely illite is addressed in most safety assessments by using simple semi-empirical kinetic models, but this approach contrasts with more complex reactive-transport simulations. In the present study, reactive-transport simulations are compared with simple semi-empirical kinetic models. Results suggest that reactive-transport simulations err on the side of conservatism, but may produce unrealistic estimates of illitization. This comparison demonstrates that reactive-transport models may be carefully applied to simulate the long-term evolution of near field environment for HLW disposal.
Savage, D.*; Wilson, J.*; Benbow, S.*; Sasamoto, Hiroshi; Oda, Chie; Walker, C.*; Kawama, Daisuke*; Tachi, Yukio
Applied Clay Science, 179, p.105146_1 - 105146_10, 2019/10
Times Cited Count:13 Percentile:52.80(Chemistry, Physical)Natural systems evidence for the effects of temperature and the activity of aqueous silica upon montmorillonite stability was evaluated. Thermodynamic modeling using three different TDBs shows that stability fields for montmorillonite exist from 0 to 140 C, but at low values of silica activity, a stability field for illite replaces that for montmorillonite. Pore fluid chemical and mineralogical data for sediments from ODP sites from offshore Japan show a trend from montmorillonite + amorphous silica stability at temperatures up to 60
C, but at low values of silica activity, a stability field for illite replaces that for montmorillonite. Pore fluid chemical and mineralogical data for sediments from ODP sites from offshore Japan show a trend from montmorillonite + amorphous silica stability at temperatures up to 60 C to that for illite + quartz at higher temperatures. However, even over very long timescales (
C to that for illite + quartz at higher temperatures. However, even over very long timescales (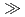 1 Ma), smectite does not transform to illite under thermodynamically-favourable conditions at temperatures less than 80
1 Ma), smectite does not transform to illite under thermodynamically-favourable conditions at temperatures less than 80 C.
C.