Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Sugo, Yumi; Kitatsuji, Yoshihiro; Kirishima, Akira*; Kimura, Takaumi; Choppin, G. R.*
Analytical Sciences, 23(6), p.727 - 731, 2007/06
Times Cited Count:63 Percentile:87.33(Chemistry, Analytical)Water-soluble ligands, tetramethyl-diglycolamide (TMDGA), tetraethyl-diglycolamide (TEDGA), tetrapropyl-diglycolamide (TPDGA) and dipropyl-diglycolamic acid (DPDGAc) were prepared and their abilities to complex with the metal cations were investigated. These results indicate that the DGA series and DPDGAc have stronger complexing ability with Am(III) and Pu(IV) than comparable carboxylic and aminopolycarboxylic acids. Among these ligands, the trend of the strength of their complexing ability is TPDGA  TEDGA
TEDGA  TMDGA
TMDGA  DPDGAc. TPDGA has significant loss to the extraction solvent due to its high hydrophobicity. It is evident from the present work that TEDGA is the best stripping reagent not only for An(III), (IV) but also Ca(II), Sc(III), Y(III), Zr(IV), La(III), Hf(IV), and Bi(III).
DPDGAc. TPDGA has significant loss to the extraction solvent due to its high hydrophobicity. It is evident from the present work that TEDGA is the best stripping reagent not only for An(III), (IV) but also Ca(II), Sc(III), Y(III), Zr(IV), La(III), Hf(IV), and Bi(III).
Sasaki, Yuji; Suzuki, Hideya*; Sugo, Yumi; Kimura, Takaumi; Choppin, G. R.*
Chemistry Letters, 35(3), p.256 - 257, 2006/03
Times Cited Count:36 Percentile:68.07(Chemistry, Multidisciplinary)Novel water-soluble organic ligands, N,N,N',N'-tetramethyl-diglycolamide (TMDGA), N,N,N',N'-tetraethyl-diglycolamide (TEDGA), N,N,N',N'-tetrapropyl-diglycolamide (TPDGA) and N,N-dipropyl-diglycolamic acid (DPDGAc), were synthesized and examined for complexation with An(III) and An(IV). These reagents are readily soluble in water and form more stable complexes with Pu(IV) and Am(III) than does EDTA of hexadentate ligand. TEDGA and TPDGA can back-extract effectively An into the aqueous phase. In addition, the An-complex in the aqueous phase can be dissociated and the TEDGA can be separated from the An and recovered by extraction using a polar organic solvent.
Billard, I.*; Ansoborlo, E.*; Apperson, K.*; Arpigny, S.*; Azenha, M.-E.*; Birch, D.*; Bros, P.*; Burrows, H. D.*; Choppin, G. R.*; Couston, L.*; et al.
Applied Spectroscopy, 57(8), p.1027 - 1038, 2003/08
Times Cited Count:50 Percentile:88.23(Instruments & Instrumentation)no abstracts in English
Sasaki, Yuji; Choppin, G. R.*
Journal of Radioanalytical and Nuclear Chemistry, 246(2), p.267 - 273, 2000/11
Times Cited Count:38 Percentile:89.75(Chemistry, Analytical)no abstracts in English

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO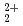 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Rai, D.*; Rao, L.*; Weger, H. T.*; Felmy, A. R.*; Choppin, G. R.*; Yui, Mikazu
JNC TN8400 99-009, 115 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Th(IV), U(IV), Np(IV), and Pu(IV) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(IV) species was lacking, the data were selected based on chemical analogy to other tetravalent actinides. ln this study, the Pitzer ion-interaction model is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Kimura, Takaumi; Kato, Yoshiharu; Takeishi, Hideyo; Takahashi, Yoshio*; Minai, Yoshitaka*; Choppin, G. R.*
Proceedings of OECD/NEA Workshop on Evaluation of Speciation Technology, p.61 - 81, 1999/00
no abstracts in English
Sasaki, Yuji; Choppin, G. R.*
Journal of Radioanalytical and Nuclear Chemistry, 222(1-2), p.271 - 274, 1997/00
Times Cited Count:15 Percentile:90.13(Chemistry, Analytical)no abstracts in English
Sasaki, Yuji; Choppin, G. R.*
Journal of Radioanalytical and Nuclear Chemistry, 207(2), p.383 - 394, 1996/07
Times Cited Count:53 Percentile:96.15(Chemistry, Analytical)no abstracts in English
Sasaki, Yuji; Choppin, G. R.*
Analytical Sciences, 12(2), p.225 - 230, 1996/04
Times Cited Count:175 Percentile:98.64(Chemistry, Analytical)no abstracts in English
Kimura, Takaumi; Choppin, G. R.*; ; Yoshida, Zenko
Radiochimica Acta, 72, p.61 - 64, 1996/00
no abstracts in English
Kimura, Takaumi; ; G.Meinrath*; Yoshida, Zenko; Choppin, G. R.*
JAERI-Conf 95-005, Vol.2, 0, p.473 - 485, 1995/03
no abstracts in English
Kimura, Takaumi; Choppin, G. R.*
Journal of Alloys and Compounds, 213-214, p.313 - 317, 1994/00
Times Cited Count:264 Percentile:99.75(Chemistry, Physical)no abstracts in English
Yonezawa, Chushiro; Choppin, G. R.*
Journal of Radioanalytical and Nuclear Chemistry, 134(1), p.233 - 239, 1989/00
Times Cited Count:29 Percentile:92.49(Chemistry, Analytical)no abstracts in English