Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ikeda, Atsushi; Tsushima, Satoru*; Takao, Koichiro*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; Yaita, Tsuyoshi; Hennig, C.*
Inorganic Chemistry, 48(24), p.11779 - 11787, 2009/12
Times Cited Count:34 Percentile:79.72(Chemistry, Inorganic & Nuclear)The electrochemical behavior and complex structure of Np carbonato complexes have been investigated in aqueous Na CO
CO and Na
and Na CO
CO /NaOH solutions at different oxidation states by using cyclic voltammetry, X-ray absorption spectroscopy, and density functional theory calculations. The end-member complexes of penta- and hexavalent Np in 1.5 M Na
/NaOH solutions at different oxidation states by using cyclic voltammetry, X-ray absorption spectroscopy, and density functional theory calculations. The end-member complexes of penta- and hexavalent Np in 1.5 M Na CO
CO with pH = 11.7 have been determined as a transdioxo neptunyl tricarbonato complex, [NpO
with pH = 11.7 have been determined as a transdioxo neptunyl tricarbonato complex, [NpO (CO
(CO )
) ]
]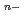 (
( = 5 for Np
= 5 for Np , and 4 for Np
, and 4 for Np ). In contrast, the electrochemical oxidation of Np
). In contrast, the electrochemical oxidation of Np in a highly basic carbonate solution of 2.0M Na
in a highly basic carbonate solution of 2.0M Na CO
CO /1.0M NaOH (pH
/1.0M NaOH (pH  13) yielded a stable heptavalent Np complex of [Np
13) yielded a stable heptavalent Np complex of [Np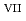 O
O (OH)
(OH) ]
] , indicating that the oxidation reaction from Np
, indicating that the oxidation reaction from Np to Np
to Np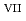 in the carbonate solution involves a drastic structural rearrangement from the transdioxo configuration to a square-planar-tetraoxo configuration, as well as exchanging the coordinating anions from carbonate ions (CO
in the carbonate solution involves a drastic structural rearrangement from the transdioxo configuration to a square-planar-tetraoxo configuration, as well as exchanging the coordinating anions from carbonate ions (CO
 ) to hydroxide ions (OH
) to hydroxide ions (OH ).
).
Ikeda, Atsushi; Hennig, C.*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; Yaita, Tsuyoshi
ESRF Highlights 2008, p.99 - 100, 2009/02
Structural arrangement of Np species has been investigated in various aqueous solutions by synchrotron-based X-ray absorption fine structure (XAFS) spectroscopy. The obtained results revealed that Np(IV) dominantly forms a spherically coordinated decahydrate complex, [Np(H O)
O)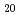 ]
] , while Np(V) and -(VI) form a pentahydrate neptunyl complex, [NpO
, while Np(V) and -(VI) form a pentahydrate neptunyl complex, [NpO (H
(H O)
O) ]
] .
.
Ikeda, Atsushi; Hennig, C.*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; Yaita, Tsuyoshi
Inorganic Chemistry, 47(18), p.8294 - 8305, 2008/09
Times Cited Count:82 Percentile:68.9(Chemistry, Inorganic & Nuclear)Electrochemical and complexation properties of neptunium (Np) are investigated in aqueous perchlorate and nitrate solutions by means of cyclic voltammetry, bulk electrolysis, UV-visible absorption and Np L -edge X-ray absorption spectroscopies. The redox reactions of Np(III)/Np(IV) and Np(V)/Np(VI) couples are reversible or quasi-reversible, while the electrochemical reaction between Np(III/IV) and Np(V/VI) is irreversible because they undergo the structural rearrangement from spherical coordinating ions (Np
-edge X-ray absorption spectroscopies. The redox reactions of Np(III)/Np(IV) and Np(V)/Np(VI) couples are reversible or quasi-reversible, while the electrochemical reaction between Np(III/IV) and Np(V/VI) is irreversible because they undergo the structural rearrangement from spherical coordinating ions (Np and Np
and Np ) to transdioxo neptunyl ions (NpO
) to transdioxo neptunyl ions (NpO
 , n = 1 for Np(V) and 2 for Np(VI)). A detailed analysis on extended X-ray absorption fine structure (EXAFS) spectra suggests that Np(IV) forms a decaaquo complex of [Np(H
, n = 1 for Np(V) and 2 for Np(VI)). A detailed analysis on extended X-ray absorption fine structure (EXAFS) spectra suggests that Np(IV) forms a decaaquo complex of [Np(H O)
O) ]
] in 1.0 M HClO
in 1.0 M HClO , while Np(V) and -(VI) exist dominantly as pentaaquo neptunyl complexes, [NpO
, while Np(V) and -(VI) exist dominantly as pentaaquo neptunyl complexes, [NpO (H
(H O)
O) ]
] (n = 1 for Np(V) and 2 for Np(VI)).
(n = 1 for Np(V) and 2 for Np(VI)).
Ikeda, Atsushi; Hennig, C.*; Rossberg, A.*; Tsushima, Satoru*; Scheinost, A. C.*; Bernhard, G.*
Analytical Chemistry, 80(4), p.1102 - 1110, 2008/01
Times Cited Count:25 Percentile:60.21(Chemistry, Analytical)A multitechnique approach using extended X-ray absorption fine structure spectroscopy based on iterative transformation factor analysis, UV-visible absorption spectroscopy, and quantum chemical calculations has been performed for identifying the complex structure of individual U(VI) nitrate species.
Ikeda, Atsushi; Hennig, C.*; Tsushima, Satoru*; Rossberg, A.*; Scheinost, A. C.*; Bernhard, G.*; Yaita, Tsuyoshi
no journal, ,
Neptunium (93Np) is one of the most problematic nuclides in the nuclear fuel reprocessing process and the following radioactive waste disposal because of its chemical similarity to the fissile nuclides of uranium (U) and plutonium (Pu). Proper understanding of the behavior of Np in the reprocessing process or in the migration process on the geological disposal of radioactive wastes requires vast fundamental information about the chemical properties of Np in solutions. In the present study, Np solution samples with different oxidation states are electrochemically prepared in aqueous perchlorate, nitrate, and carbonate solutions, and the complex structure of Np species in the sample solutions are determined by EXAFS spectroscopy and DFT calculations. The obtained results reveal the structural difference between different Np oxidation states, as well as the different coordination behavior in each solution system.