Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
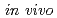 lung monitoring of enriched uranium; Results of an international comparison
lung monitoring of enriched uranium; Results of an international comparisonBroggio, D.*; Bento, J.*; Caldeira, M.*; Cardenas-Mendez, E.*; Farah, J.*; Fonseca, T.*; Konvalinka, C.*; Liu, L.*; Perez, B.*; Capello, K.*; et al.
Radiation Measurements, 47(7), p.492 - 500, 2012/07
Times Cited Count:23 Percentile:83.88(Nuclear Science & Technology)Wan, L. K.*; Peng, J.*; Lin, M.; Muroya, Yusa*; Katsumura, Yosuke*; Fu, H. Y.*
Radiation Physics and Chemistry, 81(5), p.524 - 530, 2012/05
Times Cited Count:16 Percentile:75.38(Chemistry, Physical)Rate constants of common crown ethers ((C H
H O)
O) , n=4, 5, 6) and their model compound, 1,4-dioxane(6C2) with some important oxidative radicals,
, n=4, 5, 6) and their model compound, 1,4-dioxane(6C2) with some important oxidative radicals,  OH, SO
OH, SO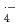 , and NO
, and NO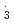 were determined in various aqueous solutions using pulse radiolysis and laser photolysis techniques. The rate constants for 6C2 and crown ethers with
were determined in various aqueous solutions using pulse radiolysis and laser photolysis techniques. The rate constants for 6C2 and crown ethers with  OH and SO
OH and SO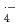 increase with the number of H-atom in the ethers, indicating that H-abstraction is dominant reaction between crown ethers and these two radicals. The presence of cations in solution has negligible effect on the rate constants for
increase with the number of H-atom in the ethers, indicating that H-abstraction is dominant reaction between crown ethers and these two radicals. The presence of cations in solution has negligible effect on the rate constants for  OH and SO
OH and SO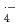 . However, for NO
. However, for NO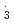 , the rate constants is not in proportional to H-atom number in crown ethers, and the 12-crown-4 is most reactive compared with the others. For the studied crown ethers, the rate constants of these oxidative radicals have the order:
, the rate constants is not in proportional to H-atom number in crown ethers, and the 12-crown-4 is most reactive compared with the others. For the studied crown ethers, the rate constants of these oxidative radicals have the order:  OH
OH  SO
SO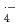
 NO
NO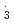 . This is the first report on the kinetic behavior of crown ethers with NO
. This is the first report on the kinetic behavior of crown ethers with NO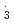 , and it would be helpful for the understanding of stability of crown ethers in the nuclear fuel reprocessing.
, and it would be helpful for the understanding of stability of crown ethers in the nuclear fuel reprocessing.
G mez-Ros, J.-M.*; de Carlan, L.*; Franck, D.*; Gualdrini, G.*; Lis, M.*; L
mez-Ros, J.-M.*; de Carlan, L.*; Franck, D.*; Gualdrini, G.*; Lis, M.*; L pez, M. A.*; Moraleda, M.*; Zankl, M.*; Badal, A.*; Capello, K.*; et al.
pez, M. A.*; Moraleda, M.*; Zankl, M.*; Badal, A.*; Capello, K.*; et al.
Radiation Measurements, 43(2-6), p.510 - 515, 2008/02
Times Cited Count:25 Percentile:82.43(Nuclear Science & Technology)This communication summarizes the results concerning the Monte Carlo modeling of Germanium detectors for the measurement of low energy photons arising from the "International comparison on MC modeling for in vivo measurement of Americium in a knee phantom" organized within the EU Coordination Action CONRAD (Coordinated Network for Radiation Dosimetry) as a joint initiative of EURADOS working groups 6 (computational dosimetry) and 7 (internal dosimetry).
Peng, J.; Wan, L. K.*; Lin, M.; Yan, Y.*; Muroya, Yusa*; Katsumura, Yosuke
no journal, ,
Wan, L. K.*; Peng, J.; Lin, M.; Muroya, Yusa*; Katsumura, Yosuke
no journal, ,
The transient absorption spectra of crown ethers in aqueous solution are studied using KrF (248 nm) laser photolysis. Moreover, the rate constants for the reaction of SO
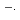 radicals with crown ethers are determined. By laser photolysis, the rate constants of the reactions of SO
radicals with crown ethers are determined. By laser photolysis, the rate constants of the reactions of SO
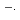 radical with 18-crown-6(18C6), 15-crown-5(15C5) and 1,4-dioxane, have been determined to be 2.5
radical with 18-crown-6(18C6), 15-crown-5(15C5) and 1,4-dioxane, have been determined to be 2.5 10
10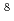 , 2.2
, 2.2 10
10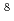 and 4.2
and 4.2 10
10 M
M s
s , respectively. The values increase with increasing the size of the ethers and K
, respectively. The values increase with increasing the size of the ethers and K and Na
and Na has no obvious effect on the reactivity.
has no obvious effect on the reactivity.