Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Tc-radiopharmaceuticals obtained from
Tc-radiopharmaceuticals obtained from  Mo produced by
Mo produced by 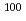 Mo(n,2n)
Mo(n,2n) Mo and fission of
Mo and fission of  U
UHashimoto, Kazuyuki; Nagai, Yasuki; Kawabata, Masako; Sato, Nozomi*; Hatsukawa, Yuichi; Saeki, Hideya; Motoishi, Shoji*; Ota, Masayuki; Konno, Chikara; Ochiai, Kentaro; et al.
Journal of the Physical Society of Japan, 84(4), p.043202_1 - 043202_4, 2015/04
Times Cited Count:8 Percentile:53(Physics, Multidisciplinary)Ishii, Yasuo; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Tome, Hayato; Nishinaka, Ichiro; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; et al.
Chemistry Letters, 37(3), p.288 - 289, 2008/03
Times Cited Count:20 Percentile:55.03(Chemistry, Multidisciplinary)We have investigated cation-exchange behavior of Rf together with the lighter homologues of the group-4 elements Zr and Hf, and the tetravalent pseudo-homologue Th, in HF/HNO solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The
solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The 
 values of Zr, Hf, Th and Rf in HF/0.1 M HNO
values of Zr, Hf, Th and Rf in HF/0.1 M HNO were decreased with increasing the concentration of the fluoride ion [F
were decreased with increasing the concentration of the fluoride ion [F ], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr
], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr  Hf
Hf  Rf
Rf  Th.
Th.
Nagame, Yuichiro; Toyoshima, Atsushi; Ishii, Yasuo; Tsukada, Kazuaki; Asai, Masato; Tome, Hayato; Kasamatsu, Yoshitaka; Nishinaka, Ichiro; Sato, Tetsuya; Haba, Hiromitsu*; et al.
no journal, ,
We present a study of fluoride complex formation of Rf at Japan Atomic Energy Agency (JAEA). Anion- and cation-exchange behavior of Rf was systematically investigated in HF/HNO mixed solution together with the lighter homologues Zr and Hf using the automated ion-exchange separation apparatus coupled with the detection system for alpha spectroscopy. We unequivocally determined the species of Rf on the binding sites of the resin as the hexafluoro complex [RfF
mixed solution together with the lighter homologues Zr and Hf using the automated ion-exchange separation apparatus coupled with the detection system for alpha spectroscopy. We unequivocally determined the species of Rf on the binding sites of the resin as the hexafluoro complex [RfF ]
] . It is found that there is about two-orders of magnitude difference in the fluoride ion concentration between Rf and the homologues for the formation of the hexafluoro complexes. The result clearly demonstrates that the formation of the fluoride complexes of Rf is much weaker than those of the homologues Zr and Hf.
. It is found that there is about two-orders of magnitude difference in the fluoride ion concentration between Rf and the homologues for the formation of the hexafluoro complexes. The result clearly demonstrates that the formation of the fluoride complexes of Rf is much weaker than those of the homologues Zr and Hf.
 Mo using accelerator neutrons and thermochromatographic separation of
Mo using accelerator neutrons and thermochromatographic separation of  Tc
TcKawabata, Masako*; Nagai, Yasuki; Hashimoto, Kazuyuki; Hatsukawa, Yuichi; Motoishi, Shoji*; Saeki, Hideya*; Sato, Nozomi*; Ota, Akio*; Shiina, Takayuki*; Kawauchi, Yukimasa*; et al.
no journal, ,
no abstracts in English