Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ishii, Yasuo; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Tome, Hayato; Nishinaka, Ichiro; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; et al.
Chemistry Letters, 37(3), p.288 - 289, 2008/03
Times Cited Count:20 Percentile:55.03(Chemistry, Multidisciplinary)We have investigated cation-exchange behavior of Rf together with the lighter homologues of the group-4 elements Zr and Hf, and the tetravalent pseudo-homologue Th, in HF/HNO solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The
solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The 
 values of Zr, Hf, Th and Rf in HF/0.1 M HNO
values of Zr, Hf, Th and Rf in HF/0.1 M HNO were decreased with increasing the concentration of the fluoride ion [F
were decreased with increasing the concentration of the fluoride ion [F ], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr
], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr  Hf
Hf  Rf
Rf  Th.
Th.
 solutions
solutionsToyoshima, Atsushi; Haba, Hiromitsu*; Tsukada, Kazuaki; Asai, Masato; Akiyama, Kazuhiko*; Goto, Shinichi*; Ishii, Yasuo; Nishinaka, Ichiro; Sato, Tetsuya; Nagame, Yuichiro; et al.
Radiochimica Acta, 96(3), p.125 - 134, 2008/03
Times Cited Count:28 Percentile:85.1(Chemistry, Inorganic & Nuclear)Formation of an anionic fluoride-complex of element 104, rutherfordium (Rf) produced in the  Cm(
Cm( O,5n)
O,5n) Rf reaction was studied by an anion-exchange method based on an atom-at-a-time scale. It was found that the hexafluoro complex of Rf, [RfF
Rf reaction was studied by an anion-exchange method based on an atom-at-a-time scale. It was found that the hexafluoro complex of Rf, [RfF ]
] , was formed in the studied fluoride ion concentrations of 0.0005 - 0.013 M. Formation of [RfF
, was formed in the studied fluoride ion concentrations of 0.0005 - 0.013 M. Formation of [RfF ]
] was significantly different from that of the homologues Zr and Hf, [ZrF
was significantly different from that of the homologues Zr and Hf, [ZrF ]
] and [HfF
and [HfF ]
] ; the evaluated formation constant of [RfF
; the evaluated formation constant of [RfF ]
] is at least one-order of magnitude smaller than those of [ZrF
is at least one-order of magnitude smaller than those of [ZrF ]
] and [HfF
and [HfF ]
] .
.
Nagame, Yuichiro; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Akiyama, Kazuhiko; Ishii, Yasuo; Sato, Tetsuya; Hirata, Masaru; Nishinaka, Ichiro; Ichikawa, Shinichi; et al.
Radiochimica Acta, 93(9-10), p.519 - 526, 2005/00
Times Cited Count:30 Percentile:87.1(Chemistry, Inorganic & Nuclear)no abstracts in English
Haba, Hiromitsu*; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Akiyama, Kazuhiko; Nishinaka, Ichiro; Hirata, Masaru; Yaita, Tsuyoshi; Ichikawa, Shinichi; Nagame, Yuichiro; et al.
Journal of the American Chemical Society, 126(16), p.5219 - 5224, 2004/04
Times Cited Count:43 Percentile:72.51(Chemistry, Multidisciplinary)Fluoride complexation of element 104, rutherfordium (Rf), produced in the  Cm(
Cm( O,5n)
O,5n) Rf reaction has been studied by anion-exchange chromatography on an atom-at-a-time scale. The anion-exchangechromatographic behavior of Rf was investigated in 1.9-13.9 M hydrofluoric acid together with those of the group-4 elements Zr and Hf produced in the
Rf reaction has been studied by anion-exchange chromatography on an atom-at-a-time scale. The anion-exchangechromatographic behavior of Rf was investigated in 1.9-13.9 M hydrofluoric acid together with those of the group-4 elements Zr and Hf produced in the  O-induced reactions on Ge and Gd targets, respectively. It was found that the adsorption behavior of Rf on anion-exchange resin is quite different from those of Zr and Hf, suggesting the influence of relativistic effect on the fluoride complexation of Rf.
O-induced reactions on Ge and Gd targets, respectively. It was found that the adsorption behavior of Rf on anion-exchange resin is quite different from those of Zr and Hf, suggesting the influence of relativistic effect on the fluoride complexation of Rf.
Nagame, Yuichiro; Haba, Hiromitsu*; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Goto, Shinichi*; Akiyama, Kazuhiko; Kaneko, Tetsuya; Sakama, Minoru*; Hirata, Masaru; et al.
Nuclear Physics A, 734, p.124 - 135, 2004/04
Times Cited Count:11 Percentile:56.88(Physics, Nuclear)no abstracts in English
Nagame, Yuichiro; Haba, Hiromitsu; Tsukada, Kazuaki; Asai, Masato; Akiyama, Kazuhiko; Hirata, Masaru; Nishinaka, Ichiro; Ichikawa, Shinichi; Nakahara, Hiromichi; Goto, Shinichi*; et al.
Czechoslovak Journal of Physics, 53, p.A299 - A304, 2003/00
Times Cited Count:7 Percentile:46.52(Physics, Multidisciplinary)no abstracts in English
Asai, Masato; Sakama, Minoru*; Tsukada, Kazuaki; Ichikawa, Shinichi; Haba, Hiromitsu; Nishinaka, Ichiro; Nagame, Yuichiro; Goto, Shinichi*; Akiyama, Kazuhiko; Toyoshima, Atsushi; et al.
Journal of Nuclear and Radiochemical Sciences, 3(1), p.187 - 190, 2002/06
EC and  decays of neutron-deficient americium, curium, and berkelium nuclei have been studied using the JAERI on-line isotope separator. Decay schemes of
decays of neutron-deficient americium, curium, and berkelium nuclei have been studied using the JAERI on-line isotope separator. Decay schemes of 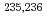 Am have been established for the first time, and proton-neutron configurations in
Am have been established for the first time, and proton-neutron configurations in 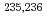 Am and
Am and 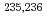 Pu have been determined.
Pu have been determined.  transitions observed in
transitions observed in 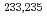 Am were found to be the favored transitions between the
Am were found to be the favored transitions between the ![$$pi 5/2^{-}[523]$$](/search/images/EROHA0JAGUXTEXT1FV4VWNJSGNOSI.gif) Nilsson states.
Nilsson states. 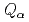 values of
values of 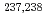 Cm have been determined. Nuclear structure, deformation, and atomic masses for these nuclei were discussed.
Cm have been determined. Nuclear structure, deformation, and atomic masses for these nuclei were discussed.
 solutions
solutionsHaba, Hiromitsu; Tsukada, Kazuaki; Asai, Masato; Goto, Shinichi*; Toyoshima, Atsushi; Nishinaka, Ichiro; Akiyama, Kazuhiko; Hirata, Masaru; Ichikawa, Shinichi; Nagame, Yuichiro; et al.
Journal of Nuclear and Radiochemical Sciences, 3(1), p.143 - 146, 2002/06
We have investigated the sorption behavior of element 104 rutherfordium (Rf) on an anion exchange resin from HCl and HNO solutions. In the HCl experiments, the distribution coefficients of Rf increase with an increase of HCl concentration from 7.0 M to 11.5 M, indicating that anionic species such as [Rf(OH)Cl
solutions. In the HCl experiments, the distribution coefficients of Rf increase with an increase of HCl concentration from 7.0 M to 11.5 M, indicating that anionic species such as [Rf(OH)Cl ]
] or [RfCl
or [RfCl ]
] are formed. This sorption behavior of Rf is typical of the group-4 elements Zr and Hf, and is quite different from that of the pseudo-homologue Th. It is also noted that the distribution coefficients decrease in the order Rf, Zr, Hf at 9.0 M HCl, which is consistent with the expected order of ionic radii. On the other hand, Rf appears to behave like Zr and Hf in 8 M HNO
are formed. This sorption behavior of Rf is typical of the group-4 elements Zr and Hf, and is quite different from that of the pseudo-homologue Th. It is also noted that the distribution coefficients decrease in the order Rf, Zr, Hf at 9.0 M HCl, which is consistent with the expected order of ionic radii. On the other hand, Rf appears to behave like Zr and Hf in 8 M HNO not like Pu and Th.
not like Pu and Th.
 Rf and
Rf and  Db in bombardments of
Db in bombardments of  Cm with
Cm with  O and
O and  F ions
F ionsNagame, Yuichiro; Asai, Masato; Haba, Hiromitsu; Goto, Shinichi*; Tsukada, Kazuaki; Nishinaka, Ichiro; Nishio, Katsuhisa; Ichikawa, Shinichi; Toyoshima, Atsushi*; Akiyama, Kazuhiko*; et al.
Journal of Nuclear and Radiochemical Sciences, 3(1), p.85 - 88, 2002/06
no abstracts in English
Haba, Hiromitsu; Tsukada, Kazuaki; Asai, Masato; Nishinaka, Ichiro; Sakama, Minoru*; Goto, Shinichi*; Hirata, Masaru; Ichikawa, Shinichi; Nagame, Yuichiro; Kaneko, Tetsuya*; et al.
Radiochimica Acta, 89(11-12), p.733 - 736, 2002/02
Times Cited Count:14 Percentile:68.97(Chemistry, Inorganic & Nuclear)no abstracts in English
 Cs
Cs*; *; *; *; *; *; *; *; Muto, Suguru*; Koizumi, Mitsuo; et al.
Physical Review B, 58(17), p.11313 - 11321, 1998/11
Times Cited Count:5 Percentile:32.45(Materials Science, Multidisciplinary)no abstracts in English
 Cs by implantation
Cs by implantation*; *; *; *; *; *; Koizumi, Mitsuo; Osa, Akihiko; Sekine, Toshiaki; *; et al.
KURRI-TR, 0, p.102 - 106, 1996/02
no abstracts in English
 Xe-implanted sources for the determination of the change of nuclear charge radius in the 81keV transition of
Xe-implanted sources for the determination of the change of nuclear charge radius in the 81keV transition of  Cs
Cs*; *; *; Miura, Taichi*; Koizumi, Mitsuo; Osa, Akihiko; Sekine, Toshiaki; *; *; *; et al.
Hyperfine Interactions (C), p.396 - 399, 1996/01
no abstracts in English
*; *; *; *; Nakahara, Hiromichi*; Nakada, Masami; Saeki, Masakatsu; Aratono, Yasuyuki
Hyperfine Interactions, 91, p.645 - 649, 1994/00
Times Cited Count:2 Percentile:20.81(Physics, Atomic, Molecular & Chemical)no abstracts in English
Yanagisawa, Ichiro*; Ezaki, Masahiro*; Ishihara, Yoshinao*; Fusaeda, Shigeki*; Mukai, Satoru*; Doi, Hideo*; Maeda, Kazuto*; Nakahara, Yutaka*
PNC TJ1214 93-001, 544 Pages, 1993/03
None
Ikeda, Yasuhiro*; Ishihara, Yoshinao*; *; *; *; Mukai, Satoru*; *
PNC TJ1214 92-002, 1340 Pages, 1992/02
None
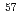 Fe-atoms after EC-decay of
Fe-atoms after EC-decay of 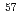 Co-labelled Co(BrO
Co-labelled Co(BrO )
)
 6H
6H O studied by coincidence Moessbauer spectroscopy
O studied by coincidence Moessbauer spectroscopyNakada, Masami; *; Nakahara, Hiromichi*; *
Hyperfine Interactions, 70, p.1241 - 1244, 1992/00
Times Cited Count:2 Percentile:17.67(Physics, Atomic, Molecular & Chemical)no abstracts in English
Tsukada, Kazuaki*; Otsuki, Tsutomu*; Sueki, Keisuke*; *; *; *; Nakahara, Hiromichi*; Shinohara, Nobuo; ; Usuda, Shigekazu; et al.
Radiochimica Acta, 51(2), p.77 - 84, 1990/00
no abstracts in English
*; *; *; *; Adachi, Junichi*; *; *; Nakazawa, Ichiro*; *; *; et al.
JAERI-M 87-091, 374 Pages, 1987/08
no abstracts in English
Nakahara, Masaumi; Tomita, Yutaka; Nomura, Kazunori; Washiya, Tadahiro
no journal, ,
Concerning the purification technology of UNH crystallization, cesium plutonium nitrate was obtained and was estimated by colorimetry, XRD, TG data. These experiments show cesium plutonium nitrate product increases with nitric acid concentration in the solution. This complex of XRD patterns was almost agreed with Cs U(NO
U(NO )
) of that. When Cs
of that. When Cs Pu(NO
Pu(NO )
) is heated for short periods, no change occurs up to 100 degrees C, after plutonium is oxidized to Cs
is heated for short periods, no change occurs up to 100 degrees C, after plutonium is oxidized to Cs PuO
PuO (NO
(NO )
) above 245 degrees C. This observed mass loss step for TG curve of cesium plutonium nitrate was about 10%.
above 245 degrees C. This observed mass loss step for TG curve of cesium plutonium nitrate was about 10%.