Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:3 Percentile:68.71(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U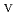
 Fe
Fe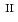
 U
U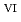 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
 , Zr, and stainless-steel
, Zr, and stainless-steelKirishima, Akira*; Akiyama, Daisuke*; Kumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Sasaki, Takayuki*; Sato, Nobuaki*
Journal of Nuclear Materials, 567, p.153842_1 - 153842_15, 2022/08
Times Cited Count:4 Percentile:78.52(Materials Science, Multidisciplinary)To understand the chemical structure and stability of nuclear fuel debris consisting of UO , Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO
, Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO -SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600
-SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600 C, in inert (Ar) or oxidative (Ar + 2% O
C, in inert (Ar) or oxidative (Ar + 2% O ) atmospheres.
) atmospheres.  Np and
Np and  Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M
Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M ssbauer spectroscopy, which provided the major uranium phase of the UO
ssbauer spectroscopy, which provided the major uranium phase of the UO  -SUS-Zr debris was the solid solution of U
-SUS-Zr debris was the solid solution of U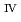 O
O (s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO
(s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO -SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
-SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
 O
O solution
solutionKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Journal of Nuclear Science and Technology, 59(8), p.961 - 971, 2022/08
Times Cited Count:2 Percentile:53.91(Nuclear Science & Technology)We investigated potential degradation of fuel debris caused by H O
O , which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H
, which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H O
O solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H
solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H O
O . Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H
. Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H O
O reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO
reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO improves the stability of fuel debris against H
improves the stability of fuel debris against H O
O reaction.
reaction.
 O
O -induced oxidative degradation of simulated fuel debris
-induced oxidative degradation of simulated fuel debrisKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Hoshasen Kagaku (Internet), (113), p.61 - 64, 2022/04
The severe accident at TEPCO's Fukushima Daiichi Nuclear Power Station resulted in generation of fuel debris. The fuel debris is in contact with water and the radiolysis of water can accelerate degradation of the debris. The analysis of particles sampled from inside or near the damaged reactors indicates the complicated compositions of the fuel debris. It is challenging to estimate the effect of water radiolysis on such a complicated material. Therefore, in this study, we investigated the potential degradation process by leaching experiments of simulated fuel debris in aqueous H O
O solution. The results show that the reaction of H
solution. The results show that the reaction of H O
O induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H
induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H O
O . These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
. These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
Komuro, Michiyasu; Kanazawa, Hiroyuki; Kokusen, Junya; Shimizu, Osamu; Honda, Junichi; Harada, Katsuya; Otobe, Haruyoshi; Nakada, Masami; Inagawa, Jun
JAEA-Technology 2021-042, 197 Pages, 2022/03
Plutonium Research Building No.1 was constructed in 1960 for the purpose of establishing plutonium handling technology and studying its basic physical properties. Radiochemical research, physicochemical research and analytical chemistry regarding solutions and solid plutonium compounds had been doing for the research program in Japan Atomic Energy Agency (JAEA). In 1964, the laboratory building was expanded and started the researching plutonium-uranium mixed fuel and reprocessing of plutonium-based fuel, playing an advanced role in plutonium-related research in Japan. Since then, the research target has been expanded to include transplutonium elements, and it has functioned as a basic research facility for actinides. The laboratory is constructed by concrete structure and it has the second floor, equipped with 15 glove boxes and 4 chemical hoods. Plutonium Research Building No.1 was decided as one of the facilities to be decommissioned by Japan Atomic Energy Agency Reform Plan in September 2014. So far, the contamination survey of the radioactive materials in the controlled area, the decontamination of glove boxes, and the consideration of the equipment dismantling procedure have been performed as planned. The radioisotope and nuclear fuel materials used in the facility have been transfer to the other facilities in JAEA. The decommissioning of the facility is proceeding with the goal of completing by decommissioning the radiation controlled area in 2026. In this report, the details of the decommissioning plan and the past achievements are reported with the several data.
Inagawa, Jun; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Nakada, Masami; Takano, Masahide; Akie, Hiroshi; Shimizu, Osamu; Komuro, Michiyasu; Oura, Hirofumi*; Nagai, Isao*; et al.
JAEA-Technology 2021-001, 144 Pages, 2021/08
Plutonium Research Building No.1 (Pu1) was qualified as a facility to decommission, and preparatory operations for decommission were worked by the research groups users and the facility managers of Pu1. The operation of transportation of whole nuclear materials in Pu1 to Back-end Cycle Key Element Research Facility (BECKY) completed at Dec. 2020. In the operation included evaluation of criticality safety for changing permission of the license for use nuclear fuel materials in BECKY, cask of the transportation, the registration request of the cask at the institute, the test transportation, formulation of plan for whole nuclear materials transportation, and the main transportation. This report circumstantially shows all of those process to help prospective decommission.
 ssbauer, infrared, and laser-induced luminescence for classifying rare-earth minerals enriched in iron-rich deposits
ssbauer, infrared, and laser-induced luminescence for classifying rare-earth minerals enriched in iron-rich depositsAoyagi, Noboru; Nguyen, T. T.*; Kumagai, Yuta; Nguyen, T. V.*; Nakada, Masami; Segawa, Yukari; Nguyen, H. T.*; Le, B. T.*
ACS Omega (Internet), 5(13), p.7096 - 7105, 2020/04
Times Cited Count:3 Percentile:15.55(Chemistry, Multidisciplinary)Segawa, Yukari; Horita, Takuma; Kitatsuji, Yoshihiro; Kumagai, Yuta; Aoyagi, Noboru; Nakada, Masami; Otobe, Haruyoshi; Tamura, Yukito*; Okamoto, Hisato; Otomo, Takashi; et al.
JAEA-Technology 2016-039, 64 Pages, 2017/03
The laboratory building No.1 for the plutonium research program (Bldg. Pu1) was chosen as one of the facilities to decommission by Japan Atomic Energy Agency Reform in September, 2013. The research groups, users of Bldg. Pu1, were driven by necessity to remove used equipment and transport nuclear fuel to other facilities from Bldg. Pu1. Research Group for Radiochemistry proactively established the Used Equipment Removal Team for the smooth operation of the removal in April, 2015. The team classified six types of work into the nature of the operation, removal of used equipment, disposal of chemicals, stabilization of mercury, stabilization of nuclear fuel, transportation of nuclear fuel and radioisotope, and survey of contamination status inside the glove boxes. These works were completed in December, 2015. This report circumstantially shows six works process, with the exception of the approval of the changes on the usage of nuclear fuel in Bldg. Pu1 to help prospective decommission.
Okamoto, Yoshihiro; Shiwaku, Hideaki; Nakada, Masami; Komamine, Satoshi*; Ochi, Eiji*; Akabori, Mitsuo
Journal of Nuclear Materials, 471, p.110 - 115, 2016/02
Times Cited Count:6 Percentile:49.29(Materials Science, Multidisciplinary)Extended X-ray Absorption Fine Structure (EXAFS) analyses were performed to evaluate REDOX (REDuction and OXidation) state of platinoid elements in simulated high-level nuclear waste glass samples prepared under different conditions of temperature and atmosphere. At first, EXAFS functions were compared with those of standard materials such as RuO . Then structural parameters were obtained from a curve fitting analysis. In addition, a fitting analysis used a linear combination of the two standard EXAFS functions of a given elements metal and oxide was applied to determine ratio of metal/oxide in the simulated glass. The redox state of Ru was successfully evaluated from the linear combination fitting results of EXAFS functions. The ratio of metal increased at more reducing atmosphere and at higher temperatures. Chemical form of rhodium oxide in the simulated glass samples was RhO
. Then structural parameters were obtained from a curve fitting analysis. In addition, a fitting analysis used a linear combination of the two standard EXAFS functions of a given elements metal and oxide was applied to determine ratio of metal/oxide in the simulated glass. The redox state of Ru was successfully evaluated from the linear combination fitting results of EXAFS functions. The ratio of metal increased at more reducing atmosphere and at higher temperatures. Chemical form of rhodium oxide in the simulated glass samples was RhO unlike expected Rh
unlike expected Rh O
O . It can be estimated rhodium behaves according with ruthenium when the chemical form is oxide.
. It can be estimated rhodium behaves according with ruthenium when the chemical form is oxide.
 revealed by
revealed by  O-NMR
O-NMRTokunaga, Yo; Nishi, Tsuyoshi; Nakada, Masami; Ito, Akinori*; Sakai, Hironori; Kambe, Shinsaku; Homma, Yoshiya*; Honda, Fuminori*; Aoki, Dai*; Walstedt, R. E.*
Physical Review B, 89(21), p.214416_1 - 214416_8, 2014/06
Times Cited Count:7 Percentile:31.93(Materials Science, Multidisciplinary)The magnetic phase transition near 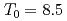 K in AmO
K in AmO has been investigated microscopically by means of
has been investigated microscopically by means of  O NMR. To avoid complexities arising from sample aging associated with the alpha decay of
O NMR. To avoid complexities arising from sample aging associated with the alpha decay of  Am, all measurements have been performed within 40 days after sample synthesis. Even during such a short period, however, a rapid change of NMR line shape has been observed at 1.5 K, suggesting that the ground state of AmO
Am, all measurements have been performed within 40 days after sample synthesis. Even during such a short period, however, a rapid change of NMR line shape has been observed at 1.5 K, suggesting that the ground state of AmO is very sensitive to disorder. We have also confirmed the loss of
is very sensitive to disorder. We have also confirmed the loss of  O NMR signal intensity over a wide temperature range below
O NMR signal intensity over a wide temperature range below 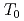 , and more than half of oxygen nuclei are undetectable at 1.5 K. This behavior reveals the persistence of slow and distributed spin fluctuations down to temperatures well below
, and more than half of oxygen nuclei are undetectable at 1.5 K. This behavior reveals the persistence of slow and distributed spin fluctuations down to temperatures well below 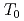 . In the paramagnetic state, strong NMR line broadening and spatially inhomogeneous spin fluctuations have been observed. The results are all indicative of short-range, spin-glass-like character for the magnetic transition in this system.
. In the paramagnetic state, strong NMR line broadening and spatially inhomogeneous spin fluctuations have been observed. The results are all indicative of short-range, spin-glass-like character for the magnetic transition in this system.
Suzuki, Chikashi; Nishi, Tsuyoshi; Nakada, Masami; Tsuru, Tomohito; Akabori, Mitsuo; Hirata, Masaru; Kaji, Yoshiyuki
Journal of Physics and Chemistry of Solids, 74(12), p.1769 - 1774, 2013/12
Times Cited Count:14 Percentile:53.53(Chemistry, Multidisciplinary)We investigated the electronic state of a CaF -type (Am,U) mixed oxide using the all-electron full potential linear augmented plane wave method and compared it with those of Am
-type (Am,U) mixed oxide using the all-electron full potential linear augmented plane wave method and compared it with those of Am O
O , AmO
, AmO , UO
, UO , and La
, and La U
U O
O . The valence of Am in the mixed oxide was close to that of Am
. The valence of Am in the mixed oxide was close to that of Am O
O and the valence of U in the mixed oxide was pentavalent. The electronic structure of AmO
and the valence of U in the mixed oxide was pentavalent. The electronic structure of AmO was different from that of Am
was different from that of Am O
O , particularly just above the Fermi level. In addition, the electronic states of Am and U in the mixed oxide were similar to those of trivalent Am and pentavalent U oxides. These electronic states reflected the high oxygen potential of AmO
, particularly just above the Fermi level. In addition, the electronic states of Am and U in the mixed oxide were similar to those of trivalent Am and pentavalent U oxides. These electronic states reflected the high oxygen potential of AmO and the heightened oxygen potential resulting from the addition of Am to UO
and the heightened oxygen potential resulting from the addition of Am to UO and also suggested the occurrence of charge transfer from Am to U in the solid solution process.
and also suggested the occurrence of charge transfer from Am to U in the solid solution process.
 U M
U M ssbauer spectroscopy
ssbauer spectroscopyTsutsui, Satoshi; Nakada, Masami
M ssbauer Spectroscopy; Applications in Chemistry, Biology, and Nanotechnology, p.123 - 140, 2013/10
ssbauer Spectroscopy; Applications in Chemistry, Biology, and Nanotechnology, p.123 - 140, 2013/10
no abstracts in English
Okamoto, Yoshihiro; Nakada, Masami; Akabori, Mitsuo; Komamine, Satoshi*; Fukui, Toshiki*; Ochi, Eiji*; Nitani, Hiroaki*; Nomura, Masaharu*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 81(7), p.543 - 546, 2013/07
Times Cited Count:7 Percentile:17.93(Electrochemistry)The molten state of simulated high-level waste glass and the behavior of ruthenium element in the melt were investigated by using synchrotron radiation based X-ray imaging technique. Melting, generating and moving of bubbles, condensation and sedimentation of ruthenium element were observed dynamically in continuous 12-bit gray-scale images from the CCD camera. X-ray intensity was obtained easily by digitizing gray-scale values in the image. The existence of ruthenium element is emphasized as a black color in the CCD image at X-ray energy higher than the Ru K-absorption edge. Position sensitive imaging X-ray absorption fine structure (XAFS) measurement was also performed to clarify the chemical state of ruthenium element in the melt.
Okamoto, Yoshihiro; Nakada, Masami; Akabori, Mitsuo; Komamine, Satoshi*; Fukui, Toshiki*; Ochi, Eiji*; Nitani, Hiroaki*; Nomura, Masaharu*
Proceedings of 4th Asian Conference on Molten Salt Chemistry and Technology & 44th Symposium on Molten Salt Chemistry, Japan, p.47 - 52, 2012/09
The molten state of the simulated high-level waste glass and the behavior of ruthenium element in the melt were investigated by using synchrotron radiation based X-ray imaging technique. Melting, generating and moving of bubbles, condensation and sedimentation of ruthenium element were observed dynamically in continuous 12-bit gray-scale images from the CCD camera. The existence of ruthenium in the X-ray CCD image was emphasized over the energy of Ru K-absorption edge. X-ray intensity was obtained easily by digitalizing gray-scale values in the image. Position sensitive imaging X-ray absorption fine structure (XAFS) measurement was performed to clarify the chemical state of ruthenium element in the melt.
Okamoto, Yoshihiro; Nakada, Masami; Akabori, Mitsuo; Shiwaku, Hideaki; Komamine, Satoshi*; Fukui, Toshiki*; Ochi, Eiji*; Nitani, Hiroaki*; Nomura, Masaharu*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 11(2), p.127 - 132, 2012/06
Distribution and the chemical state of Ru element in the simulated high-level waste glass were examined by using the synchrotron radiation based X-ray imaging technique. In this technique, a direct X-ray CCD camera is used in place of an ion chamber. Position sensitive X-ray absorption spectra were obtained by analyzing gray scale in images of the X-ray CCD camera. At first, we measured a test sample containing RuO and Ru metal powder. We successfully obtained information on the Ru distribution in the sample. In addition, the chemical state (oxide or metal ?) of each small Ru-rich spot was evaluated by the corresponding position sensitive XAFS spectrum. The imaging XAFS technique was applied to some simulated high-level waste glass samples. The Ru distribution of the glass sample and their chemical state were confirmed by image analyses. It can be seen that Ru element scattered in the glass sample exists as oxide RuO
and Ru metal powder. We successfully obtained information on the Ru distribution in the sample. In addition, the chemical state (oxide or metal ?) of each small Ru-rich spot was evaluated by the corresponding position sensitive XAFS spectrum. The imaging XAFS technique was applied to some simulated high-level waste glass samples. The Ru distribution of the glass sample and their chemical state were confirmed by image analyses. It can be seen that Ru element scattered in the glass sample exists as oxide RuO .
.
Suzuki, Chikashi; Nishi, Tsuyoshi; Nakada, Masami; Akabori, Mitsuo; Hirata, Masaru; Kaji, Yoshiyuki
Journal of Physics and Chemistry of Solids, 73(2), p.209 - 216, 2012/02
Times Cited Count:26 Percentile:69.41(Chemistry, Multidisciplinary)The authors investigated theoretically core-hole effects on X-ray absorption near-edge structures (XANES) of Np and Am L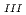 in neptunium dioxide (NpO
in neptunium dioxide (NpO ) and americium dioxide (AmO
) and americium dioxide (AmO ) with CaF
) with CaF -type crystal lattices using the all-electron full-potential linearized augmented plane-wave (FP-LAPW) method. The peak creation mechanism of XANES was shown by examining the electronic structures of these oxides, which indicated that core-hole screening was more marked for AmO
-type crystal lattices using the all-electron full-potential linearized augmented plane-wave (FP-LAPW) method. The peak creation mechanism of XANES was shown by examining the electronic structures of these oxides, which indicated that core-hole screening was more marked for AmO than for NpO
than for NpO because of the difference in the charge transfer between these oxides. Furthermore, the results of charge density analysis suggested that the white line was assigned to the quasi-bound state composed of the localized Np d or Am d components and O components, and that the tail structure was created as a result of delocalized standing waves between the Np or Am atoms.
because of the difference in the charge transfer between these oxides. Furthermore, the results of charge density analysis suggested that the white line was assigned to the quasi-bound state composed of the localized Np d or Am d components and O components, and that the tail structure was created as a result of delocalized standing waves between the Np or Am atoms.
Tokunaga, Yo; Sakai, Hironori; Kambe, Shinsaku; Chudo, Hiroyuki; Osaka, Masahiko; Miwa, Shuhei; Nishi, Tsuyoshi; Nakada, Masami; Ito, Akinori; Homma, Yoshiya*
Materials Research Society Symposium Proceedings, Vol.1444, p.149 - 158, 2012/00
Times Cited Count:0 Percentile:0.11(Materials Science, Multidisciplinary)Besides the importance of AnO series (An; U, Np, Pu, Am) as a nuclear fuel, the magnetic properties of these compounds at low temperatures are particularly interesting. Their surprisingly varied physical properties continue to be of interest for both theory and experiment. In this study, we have performed NMR studies for the series of actinide dioxides. On the basis of
series (An; U, Np, Pu, Am) as a nuclear fuel, the magnetic properties of these compounds at low temperatures are particularly interesting. Their surprisingly varied physical properties continue to be of interest for both theory and experiment. In this study, we have performed NMR studies for the series of actinide dioxides. On the basis of  O-NMR studies, exotic magnetic orderings associated with multipole degrees of freedom on 5
O-NMR studies, exotic magnetic orderings associated with multipole degrees of freedom on 5 electrons have been identified in UO
electrons have been identified in UO (dipolar + quadrupolar ordering) and NpO
(dipolar + quadrupolar ordering) and NpO (octupolar + quadrupolar ordering), in contrast with the non-magnetic ground state of PuO
(octupolar + quadrupolar ordering), in contrast with the non-magnetic ground state of PuO . In AmO
. In AmO , our
, our  O-NMR data provide the first microscopic evidence for a phase transition as a bulk property in this system.
O-NMR data provide the first microscopic evidence for a phase transition as a bulk property in this system.
 Am
Am )O
)O
Nishi, Tsuyoshi; Nakada, Masami; Suzuki, Chikashi; Shibata, Hiroki; Okamoto, Yoshihiro; Akabori, Mitsuo; Hirata, Masaru
Journal of Nuclear Materials, 418(1-3), p.311 - 312, 2011/11
Times Cited Count:20 Percentile:81.61(Materials Science, Multidisciplinary)The XAFS measurements at U-L and Am-L
and Am-L absorption edge of (U
absorption edge of (U Am
Am )O
)O were performed in transmission mode. Moreover, to clarify the valence state of Am in (U,Am)O
were performed in transmission mode. Moreover, to clarify the valence state of Am in (U,Am)O , the XANES spectrum of Am-L
, the XANES spectrum of Am-L absorption edge of (U
absorption edge of (U Am
Am )O
)O was verified using those of Am-L
was verified using those of Am-L absorption edge of AmO
absorption edge of AmO and Am
and Am O
O . It was found that the XANES spectrum of the Am-L
. It was found that the XANES spectrum of the Am-L edge of (U
edge of (U Am
Am )O
)O is in good accordance with that of Am
is in good accordance with that of Am O
O . Thus, Am in (U
. Thus, Am in (U Am
Am )O
)O is almost trivalent state.
is almost trivalent state.
 ; Comparison with UO
; Comparison with UO and NpO
and NpO
Tokunaga, Yo; Nishi, Tsuyoshi; Kambe, Shinsaku; Nakada, Masami; Homma, Yoshiya*; Sakai, Hironori; Chudo, Hiroyuki
Journal of the Physical Society of Japan, 80(Suppl.A), p.SA110_1 - SA110_3, 2011/07
Times Cited Count:2 Percentile:20.16(Physics, Multidisciplinary)We have successfully performed an  O NMR for the first time on Americium dioxide (AmO
O NMR for the first time on Americium dioxide (AmO ). We observed a drastic broadening of the NMR spectrum, after a sudden drop of NMR signal intensity below
). We observed a drastic broadening of the NMR spectrum, after a sudden drop of NMR signal intensity below 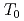 . These data provide the first microscopic evidence for a phase transition as a bulk property in this system. In the ordered state, we found a spectrum with a triangular line shape, which resembles neither that of UO
. These data provide the first microscopic evidence for a phase transition as a bulk property in this system. In the ordered state, we found a spectrum with a triangular line shape, which resembles neither that of UO nor NpO
nor NpO . It is also suggested that there is considerable damage to the sample induced by self-radiation effects from the alpha decay of
. It is also suggested that there is considerable damage to the sample induced by self-radiation effects from the alpha decay of  Am.
Am.
Amamoto, Ippei; Mitamura, Naoki*; Tsuzuki, Tatsuya*; Takasaki, Yasushi*; Shibayama, Atsushi*; Yano, Tetsuji*; Nakada, Masami; Okamoto, Yoshihiro
Proceedings of 13th International Conference on Environmental Remediation and Radioactive Waste Management (ICEM 2010) (CD-ROM), p.503 - 508, 2010/10
The main objective of this development is to recycle the purified eutectic medium of the pyroreprocessing, delaying its disposal for as long as possible. We have introduced the simple filtration method to remove the rare earth element (REE) particles which were formed due to the conversion of REE chlorides to phosphates. Here, the iron phosphate glass is used as a filtration medium for the removal of FP particles. However, some soluble FP such as compounds of alkali-metals, alkaline-earth metals, etc. still remain in the eutectic medium. This time around, on an experimental basis, the iron phosphate glass has been used as a sorbent instead, to remove the soluble FP. We have obtained some positive results and have intention to incorporate it into the spent electrolyte recycle process as a part of the FP separation and immobilization system.