Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Machida, Shinichi*; Hattori, Takanori; Nakano, Satoshi*; Sano, Asami; Funakoshi, Kenichi*; Abe, Jun*
Koatsuryoku No Kagaku To Gijutsu, 34(3), p.134 - 142, 2024/09
A diamond anvil cell (DAC) for high-pressure neutron diffraction experiments has been developed at the PLANET beamline, Materials and Life Science Experimental Facility, in J-PARC. The conically supported diamond anvils were used for high-pressure generation. We succeeded in obtaining the neutron data for D O ice up to 69.4 GPa. In addition, the gasket materials suitable for the neutron diffraction measurements were investigated. 11 kinds of alloys were tested and SUS304, Inconel718 and M2052 (73Mn-20Cu-5Ni-2Fe, at%) alloys showed excellent performance. Especially, M2052 null-matrix alloy has proven to be useful for neutron diffraction experiments where the beam inevitably hits the gasket. We then obtained refinable neutron diffraction profiles in Rietveld analysis from D
O ice up to 69.4 GPa. In addition, the gasket materials suitable for the neutron diffraction measurements were investigated. 11 kinds of alloys were tested and SUS304, Inconel718 and M2052 (73Mn-20Cu-5Ni-2Fe, at%) alloys showed excellent performance. Especially, M2052 null-matrix alloy has proven to be useful for neutron diffraction experiments where the beam inevitably hits the gasket. We then obtained refinable neutron diffraction profiles in Rietveld analysis from D O ice at least up to 43.3 GPa.
O ice at least up to 43.3 GPa.
He, X.*; Kagi, Hiroyuki*; Komatsu, Kazuki*; Iizuka, Riko*; Okajima, Hajime*; Hattori, Takanori; Sano, Asami; Machida, Shinichi*; Abe, Jun*; Goto, Hirotada*; et al.
Journal of Molecular Structure, 1310, p.138271_1 - 138271_8, 2024/08
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)High-pressure responses of the O-D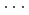 F hydrogen bonds in deuterated magnesium hydroxyfluoride were investigated using neutron powder diffraction and Raman spectroscopy. The Rietveld analysis at ambient conditions revealed a chemical formula of Mg(OD)
F hydrogen bonds in deuterated magnesium hydroxyfluoride were investigated using neutron powder diffraction and Raman spectroscopy. The Rietveld analysis at ambient conditions revealed a chemical formula of Mg(OD)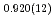 F
F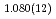 and hydroxyl group/fluorine disorder (OD/F disorder) in the crystal structure, which gave rise to two hydrogen-bonding configurations. The Rietveld analysis showed the hydrogen-bonding geometries remains up to 9.8 GPa, indicating no pressure-induced strengthening of hydrogen bonds. The Raman spectra at ambient conditions showed three hydroxyl stretching bands at 2613, 2694, and 2718 cm
and hydroxyl group/fluorine disorder (OD/F disorder) in the crystal structure, which gave rise to two hydrogen-bonding configurations. The Rietveld analysis showed the hydrogen-bonding geometries remains up to 9.8 GPa, indicating no pressure-induced strengthening of hydrogen bonds. The Raman spectra at ambient conditions showed three hydroxyl stretching bands at 2613, 2694, and 2718 cm . The high frequencies of the O-D stretching modes indicated that the hydroxyls should be involved in weak or none hydrogen-bonding interactions. Up to 20.2 GPa, the mode initially centered at 2694 cm
. The high frequencies of the O-D stretching modes indicated that the hydroxyls should be involved in weak or none hydrogen-bonding interactions. Up to 20.2 GPa, the mode initially centered at 2694 cm displayed a pressure-induced blue shift, revealing no strengthening of hydrogen bonds under compression. We discuss the existence of hydrogen bonds and the causes of the blue-shifting hydroxyls at ambient and at high pressures.
displayed a pressure-induced blue shift, revealing no strengthening of hydrogen bonds under compression. We discuss the existence of hydrogen bonds and the causes of the blue-shifting hydroxyls at ambient and at high pressures.
Nakano, Satoshi*; Sano, Asami; Hattori, Takanori; Machida, Shinichi*; Komatsu, Kazuki*; Fujihisa, Hiroshi*; Yamawaki, Hiroshi*; Goto, Yoshito*; Kikegawa, Takumi*
Inorganic Chemistry, 60(5), p.3065 - 3073, 2021/03
Times Cited Count:13 Percentile:73.31(Chemistry, Inorganic & Nuclear)X-ray and neutron diffraction analyses of ammonia borane were conducted at ambient and high pressures. The H-H distance in dihydrogen bonds was shorter than twice the van der Waals radius (2.4  ). The half of the dihydrogen bonds were broken on phase transition from AP to the first high pressure phase (HP1) at approximately 1.2 GPa as revealed by an increase in the H-H distances. On further pressure increase, all of the H-H distances became shorter than 2.4
). The half of the dihydrogen bonds were broken on phase transition from AP to the first high pressure phase (HP1) at approximately 1.2 GPa as revealed by an increase in the H-H distances. On further pressure increase, all of the H-H distances became shorter than 2.4  again, implying the pressure-induced reformation of the dihydrogen bonds. Furthermore, the HP1 transformed to the second one with the structure of
again, implying the pressure-induced reformation of the dihydrogen bonds. Furthermore, the HP1 transformed to the second one with the structure of 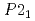 (Z = 2) at about 11 GPa. In this phase transition, the inclination of the molecule axis became larger and the number of types of dihydrogen bonds increased from 6 to 11. Just before the third transition at 18.9 GPa, the shortest dihydrogen bond decreased to 1.65
(Z = 2) at about 11 GPa. In this phase transition, the inclination of the molecule axis became larger and the number of types of dihydrogen bonds increased from 6 to 11. Just before the third transition at 18.9 GPa, the shortest dihydrogen bond decreased to 1.65  . The present study experimentally first confirmed the breakage and reformation of the dihydrogen bonds by the structural change under pressure.
. The present study experimentally first confirmed the breakage and reformation of the dihydrogen bonds by the structural change under pressure.
Komatsu, Kazuki*; Klotz, S.*; Nakano, Satoshi*; Machida, Shinichi*; Hattori, Takanori; Sano, Asami; Yamashita, Keishiro*; Irifune, Tetsuo*
High Pressure Research, 40(1), p.184 - 193, 2020/02
Times Cited Count:14 Percentile:66.41(Physics, Multidisciplinary)A new high pressure cells for neutron diffraction experiments using nano-polycrystalline anvil is presented. The cell design, off-line pressure generation tests and a gas-loading procedure for this cell are described. The performance is illustrated by powder neutron diffraction patterns of ice VII to  82 GPa. We also demonstrate the feasibility of single crystal neutron diffraction experiments of Fe
82 GPa. We also demonstrate the feasibility of single crystal neutron diffraction experiments of Fe O
O at ambient conditions using this cell and discuss the current limitation and future developments.
at ambient conditions using this cell and discuss the current limitation and future developments.
Ishii, Yusuke*; Komatsu, Kazuki*; Nakano, Satoshi*; Machida, Shinichi*; Hattori, Takanori; Sano, Asami; Kagi, Hiroyuki*
Physical Chemistry Chemical Physics, 20(24), p.16650 - 16656, 2018/06
Times Cited Count:5 Percentile:19.53(Chemistry, Physical)The structure of an aluminum layered hydroxide, boehmite ( -AlOOH), as a function of pressure was studied by using
-AlOOH), as a function of pressure was studied by using  synchrotron X-ray and neutron diffraction. Peak broadening and subsequent splitting, which are only found for hkl (h
synchrotron X-ray and neutron diffraction. Peak broadening and subsequent splitting, which are only found for hkl (h 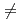 0) peaks in the X-ray diffraction patterns above 25 GPa, are explained by stacking disorder accompanied with a continuously increasing displacement of the AlO
0) peaks in the X-ray diffraction patterns above 25 GPa, are explained by stacking disorder accompanied with a continuously increasing displacement of the AlO octahedral layer along a-axis. This finding could be the first experimental result for the pressure-induced stacking disorder driven by the continuous layer displacement. The magnitude of the layer displacement was estimated from the X-ray scattering profile calculation based on the stacking disordered structure model. Hydrogen bond geometries of boehmite, obtained by structure refinements on the observed neutron diffraction patterns for deuterated sample up to 10 GPa, show linearly approaching O-D covalent and D
octahedral layer along a-axis. This finding could be the first experimental result for the pressure-induced stacking disorder driven by the continuous layer displacement. The magnitude of the layer displacement was estimated from the X-ray scattering profile calculation based on the stacking disordered structure model. Hydrogen bond geometries of boehmite, obtained by structure refinements on the observed neutron diffraction patterns for deuterated sample up to 10 GPa, show linearly approaching O-D covalent and D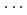 O hydrogen bond distances and they could merge below 26 GPa. The pressure-induced stacking disorder would make the electrostatic potential of hydrogen bonds asymmetric, yielding less chance for the proton-tunnelling.
O hydrogen bond distances and they could merge below 26 GPa. The pressure-induced stacking disorder would make the electrostatic potential of hydrogen bonds asymmetric, yielding less chance for the proton-tunnelling.
Iizuka, Riko*; Kagi, Hiroyuki*; Komatsu, Kazuki*; Ushijima, Daichi*; Nakano, Satoshi*; Sano, Asami; Nagai, Takaya*; Yagi, Takehiko*
Physics and Chemistry of Minerals, 38(10), p.777 - 785, 2011/12
Times Cited Count:11 Percentile:34.23(Materials Science, Multidisciplinary)The pressure responses of portlandite and the isotope effect on the phase transition were investigated at room temperature from single-crystal Raman and IR spectra and from powder X-ray diffraction using diamond anvil cells under quasi-hydrostatic conditions in a helium pressure-transmitting medium. Phase transformation and subsequent peak broadening observed from the Raman and IR spectra of Ca(OH) occurred at lower pressures than those of Ca(OD)
occurred at lower pressures than those of Ca(OD) . In contrast, no isotope effect was found on the volume and axial compressions observed from powder X-ray diffraction patterns. X-ray diffraction lines attributable to the high-pressure phase remained up to 28.5 GPa, suggesting no total amorphization in a helium pressure medium within the examined pressure region. These results suggest that the H-D isotope effect is engendered in the local environment surrounding H(D) atoms.
. In contrast, no isotope effect was found on the volume and axial compressions observed from powder X-ray diffraction patterns. X-ray diffraction lines attributable to the high-pressure phase remained up to 28.5 GPa, suggesting no total amorphization in a helium pressure medium within the examined pressure region. These results suggest that the H-D isotope effect is engendered in the local environment surrounding H(D) atoms.
Machida, Akihiko; Watanuki, Tetsu; Omura, Ayako*; Ikeda, Tomohiro*; Aoki, Katsutoshi; Nakano, Satoshi*; Takemura, Kenichi*
Solid State Communications, 151(5), p.341 - 345, 2011/03
Times Cited Count:11 Percentile:42.71(Physics, Condensed Matter)The compressibility of lanthanum (La) metal and its hydrides were measured at room temperature by high pressure synchrotron X-ray diffraction. La metal pressurized in a hydrogen medium forms a hydride with an fcc metal lattice, which likely contains hydrogen at a concentration close to 3.0. Equations of state have been determined by helium compression experiments for LaH with tetrahedral interstitial sites fully occupied with hydrogen atoms and for LaH
with tetrahedral interstitial sites fully occupied with hydrogen atoms and for LaH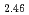 with octahedral interstitial sites partially occupied with hydrogen atoms and tetrahedral sites fully occupied. Both hydrides possess fcc metal lattices. These values are three times larger than that of La metal and are very close to each other despite the difference in hydrogen occupation states. The hardening of the metal lattice by hydrogenation is attributed predominantly to hydrogen-metal interactions at the tetrahedral sites and is most pronounced for La, which has the largest ionic radius among rare-earth metals.
with octahedral interstitial sites partially occupied with hydrogen atoms and tetrahedral sites fully occupied. Both hydrides possess fcc metal lattices. These values are three times larger than that of La metal and are very close to each other despite the difference in hydrogen occupation states. The hardening of the metal lattice by hydrogenation is attributed predominantly to hydrogen-metal interactions at the tetrahedral sites and is most pronounced for La, which has the largest ionic radius among rare-earth metals.
Omura, Ayako*; Machida, Akihiko; Watanuki, Tetsu; Aoki, Katsutoshi; Nakano, Satoshi*; Takemura, Kenichi*
Applied Physics Letters, 91(15), p.151904_1 - 151904_3, 2007/10
Times Cited Count:38 Percentile:76.97(Physics, Applied)Transparent orange yttrium hydride turns to black when illuminated by visible laser light at pressures of several gigapascals at room temperature. The marked reduction in optical transmittance extends over the infrared region, suggesting that illumination creates persistent free carriers. The opaque black sample returns to the transparent orange hydride during room-temperature annealing for a few hours. Photochromism is pronounced for the coexistent state of the metallic fcc-YH and the insulating hexagonal-YH
and the insulating hexagonal-YH state, but is depressed for the single phase of hexagonal-YH
state, but is depressed for the single phase of hexagonal-YH . The results indicate that light illumination can modify the optical and possibly electronic properties during a certain period of times.
. The results indicate that light illumination can modify the optical and possibly electronic properties during a certain period of times.
Omura, Ayako; Machida, Akihiko; Watanuki, Tetsu; Aoki, Katsutoshi; Nakano, Satoshi*; Takemura, Kenichi*
Journal of Alloys and Compounds, 446-447, p.598 - 602, 2007/10
Times Cited Count:19 Percentile:67.42(Chemistry, Physical)Pressure-induced structural change from hcp to fcc-like metal lattice was investigated for scandium hydride, ScHx, by X-ray diffraction measurement up to 60 GPa at room temperature. Hydride was prepared in a diamond anvil cell (DAC) by compressing the metal foil with fluid hydrogen. The metal foil reacted with fluid hydrogen to form ScH with an fcc metal lattice at about 4 GPa. Up to 5 GPa, fcc ScH
with an fcc metal lattice at about 4 GPa. Up to 5 GPa, fcc ScH changed for the most part to ScH
changed for the most part to ScH with a hexagonal metal lattice, which persisted to about 25 GPa together with unreacted ScH
with a hexagonal metal lattice, which persisted to about 25 GPa together with unreacted ScH . On further compression, the hcp lattice began to transform towards an fcc-like lattice. The transition completed at about 46 GPa. The successive change in diffraction pattern during the transition was quite similar to that observed for YHx, the transient state was not explained by the coexistence phase of ScH
. On further compression, the hcp lattice began to transform towards an fcc-like lattice. The transition completed at about 46 GPa. The successive change in diffraction pattern during the transition was quite similar to that observed for YHx, the transient state was not explained by the coexistence phase of ScH and ScH
and ScH but able to be interpreted in terms of the gradual change in the sequential stacking of the metal layers.
but able to be interpreted in terms of the gradual change in the sequential stacking of the metal layers.
Machida, Akihiko; Omura, Ayako; Watanuki, Tetsu; Ikeda, Takashi; Aoki, Katsutoshi; Nakano, Satoshi*; Takemura, Kenichi*
Solid State Communications, 138(9), p.436 - 440, 2006/06
Times Cited Count:53 Percentile:85.45(Physics, Condensed Matter)no abstracts in English
 at high pressure
at high pressureOmura, Ayako; Machida, Akihiko; Watanuki, Tetsu; Aoki, Katsutoshi; Nakano, Satoshi*; Takemura, Kenichi*
Physical Review B, 73(10), p.104105_1 - 104105_7, 2006/03
Times Cited Count:58 Percentile:87.08(Materials Science, Multidisciplinary)Infrared vibrational absorption spectra are measured for yttrium trihydride at room temperature and pressures up to 30 GPa. The spectral change that begins near 12 GPa is interpreted in terms of a hcp-fcc structural transition, which agrees with previous X-ray diffraction measurements. For the hydrogen vibrations in the low-pressure hcp phase, the mode Gr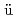 neisen parameters are derived from the observed peak frequency shifts with pressure and the reported bulk modulus. The value of 1.91 for the octahedral-site vibration is three times larger than those for the tetrahedral-site vibrations, suggesting hybridization between the hydrogen-1
neisen parameters are derived from the observed peak frequency shifts with pressure and the reported bulk modulus. The value of 1.91 for the octahedral-site vibration is three times larger than those for the tetrahedral-site vibrations, suggesting hybridization between the hydrogen-1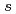 and yttrium-4
and yttrium-4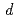 orbitals at the octahedral site. The infrared transmission spectra collapse when the high-pressure fcc phase is compressed beyond 23 GPa. The band gap abruptly closes without a structural change in the fcc metal lattice. The experimental results are contrast to the previous theoretical calculations predicting the electronic transition either with hcp-fcc structural change or in the hcp low-pressure phase without structural change. The transition mechanism is still inconclusive.
orbitals at the octahedral site. The infrared transmission spectra collapse when the high-pressure fcc phase is compressed beyond 23 GPa. The band gap abruptly closes without a structural change in the fcc metal lattice. The experimental results are contrast to the previous theoretical calculations predicting the electronic transition either with hcp-fcc structural change or in the hcp low-pressure phase without structural change. The transition mechanism is still inconclusive.
Omura, Ayako; Machida, Akihiko; Watanuki, Tetsu; Aoki, Katsutoshi; Nakano, Satoshi*; Takemura, Kenichi*
Proceedings of Joint 20th AIRAPT - 43rd EHPRG International Conference on High Pressure Science and Technology (CD-ROM), 3 Pages, 2005/06
Yttrium hydride shows a structural change from a cubic dihydride to a hexagonal trihydride by hydrogenation. This is accompanied by the metal-insulator transition. The theoretical calculation of yttrium hydride speculates that the hybridization between 4d-Y and 1s-H leads to the opening of a large energy gap. They also predict that a transition from insulator to metal occurs when the volume is reduced to about 85% of the equilibrium volume in yttrium trihydride. Recently, we performed an infrared spectroscopy of yttrium trihydride to investigate pressure-induced metallization under high pressure. The insulator-metal transition occurred with an abrupt disappearance of the optical gap of  1 eV at 23 GPa. There is a possibility of the electronic transition because no structural changes occur above 20 GPa. The electronic transition can be attributed to the delocalization of the 1s electrons bound to the H+ core ions or the rearrangement of hydrogen atoms.
1 eV at 23 GPa. There is a possibility of the electronic transition because no structural changes occur above 20 GPa. The electronic transition can be attributed to the delocalization of the 1s electrons bound to the H+ core ions or the rearrangement of hydrogen atoms.
Utsumi, Wataru; Taniguchi, Takashi*; Mizutani, Takeshi; Nishiyama, Norimasa*; Nakano, Satoshi*; Funakoshi, Kenichi*; Shimomura, Osamu
Proceedings of the 6th NIRIM International symposium on Advanced Materials (ISAM99), p.67 - 68, 1999/03
no abstracts in English