Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kobayashi, Toru; Yaita, Tsuyoshi; Suzuki, Shinichi; Shiwaku, Hideaki; Okamoto, Yoshihiro; Akutsu, Kazuhiro*; Nakano, Yoshiharu*; Fujii, Yuki*
Separation Science and Technology, 45(16), p.2431 - 2436, 2010/11
Times Cited Count:35 Percentile:71.17(Chemistry, Multidisciplinary) -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide complexes
-diphenylpyridine-2,6-dicarboxyamide complexesFujiwara, Asako; Nakano, Yoshiharu*; Yaita, Tsuyoshi; Okuno, Kenji*
Journal of Alloys and Compounds, 456(1-2), p.429 - 435, 2008/05
Times Cited Count:17 Percentile:64.5(Chemistry, Physical)The tridentate ligand  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide (DMDPhPDA) and the corresponding lanthanum complex [La(NO
-diphenylpyridine-2,6-dicarboxyamide (DMDPhPDA) and the corresponding lanthanum complex [La(NO )
) (DMDPhPDA)
(DMDPhPDA) ] have been prepared and structurally characterised. In the lanthanum complex, two DMDPhPDA molecules coordinated to La(III) in a tridentate fashion and to three nitrate ions in a bidentate fashion make the lanthanum atom 12-coordinate. The stability constants determined by spectrophotometric titration suggest that [Ln(DMDPhPDA)
] have been prepared and structurally characterised. In the lanthanum complex, two DMDPhPDA molecules coordinated to La(III) in a tridentate fashion and to three nitrate ions in a bidentate fashion make the lanthanum atom 12-coordinate. The stability constants determined by spectrophotometric titration suggest that [Ln(DMDPhPDA) ]
] is the primary product in nitrate media and [Ln(DMDPhPDA)
is the primary product in nitrate media and [Ln(DMDPhPDA) ]
] is difficult to form. However, [Ln(DMDPhPDA)
is difficult to form. However, [Ln(DMDPhPDA) ]
] could not be distinguished in
could not be distinguished in  C NMR spectra. The
C NMR spectra. The  C NMR titration results imply that a fast ligand exchange process takes place.
C NMR titration results imply that a fast ligand exchange process takes place.
 -dialkyl-2,6-pyridinedimethanaminium halide
-dialkyl-2,6-pyridinedimethanaminium halideKobayashi, Toru; Yaita, Tsuyoshi; Sugo, Yumi; Suda, Hiroki*; Suzuki, Shinji*; Fujii, Yuki*; Nakano, Yoshiharu*
Journal of Heterocyclic Chemistry, 43(3), p.549 - 557, 2006/05
Times Cited Count:5 Percentile:13.44(Chemistry, Organic) solutions using
solutions using  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide
-diphenylpyridine-2,6-dicarboxyamideShimada, Asako; Yaita, Tsuyoshi; Narita, Hirokazu; Tachimori, Shoichi; Kimura, Takaumi; Okuno, Kenji*; *
Solvent Extraction Research and Development, Japan, 11, p.1 - 10, 2004/04
The distribution ratio ( ) of Am(III) and lighter Ln(III) in the extraction with
) of Am(III) and lighter Ln(III) in the extraction with  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO solutions were determined. The D's increased with an increase of HNO
solutions were determined. The D's increased with an increase of HNO concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO
concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO concentration range (S.F.=(D
concentration range (S.F.=(D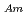 /D
/D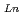 )). From 4 M HNO
)). From 4 M HNO solution with 0.5 M DMDPhPDA CHCl
solution with 0.5 M DMDPhPDA CHCl solution, the
solution, the  's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO
's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO concentration region than that has been reported so far. These
concentration region than that has been reported so far. These  's value and the S.F. were reproduced in the extraction from the metal concentration range from 10
's value and the S.F. were reproduced in the extraction from the metal concentration range from 10 M order to 10
M order to 10 M.
M.
Sakamoto, Fuminori; *; Sato, Minoru*; *; X.S.Tan*; Fujii, Yuki*
Chemical Communications, (13), p.1391 - 1392, 1998/00
no abstracts in English
Kobayashi, Toru; Yaita, Tsuyoshi; Suzuki, Shinichi; Shiwaku, Hideaki; Okamoto, Yoshihiro; Akutsu, Kazuhiro; Nakano, Yoshiharu*; Fujii, Yuki*
no journal, ,
no abstracts in English
Shiwaku, Hideaki; Akutsu, Kazuhiro; Yaita, Tsuyoshi; Okamoto, Yoshihiro; Kobayashi, Toru; Numakura, Masahiko; Nakano, Yoshiharu*; Mahara, Saori*; Fujii, Yuki*
no journal, ,
no abstracts in English
Akutsu, Kazuhiro; Shiwaku, Hideaki; Okamoto, Yoshihiro; Suzuki, Shinichi; Ikeda, Atsushi; Kobayashi, Toru*; Numakura, Masahiko; Mahara, Saori*; Nakano, Yoshiharu*; Fujii, Yuki*; et al.
no journal, ,
no abstracts in English