Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 revealed by
revealed by  O-NMR
O-NMRTokunaga, Yo; Nishi, Tsuyoshi; Nakada, Masami; Ito, Akinori*; Sakai, Hironori; Kambe, Shinsaku; Homma, Yoshiya*; Honda, Fuminori*; Aoki, Dai*; Walstedt, R. E.*
Physical Review B, 89(21), p.214416_1 - 214416_8, 2014/06
Times Cited Count:7 Percentile:31.93(Materials Science, Multidisciplinary)The magnetic phase transition near 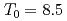 K in AmO
K in AmO has been investigated microscopically by means of
has been investigated microscopically by means of  O NMR. To avoid complexities arising from sample aging associated with the alpha decay of
O NMR. To avoid complexities arising from sample aging associated with the alpha decay of  Am, all measurements have been performed within 40 days after sample synthesis. Even during such a short period, however, a rapid change of NMR line shape has been observed at 1.5 K, suggesting that the ground state of AmO
Am, all measurements have been performed within 40 days after sample synthesis. Even during such a short period, however, a rapid change of NMR line shape has been observed at 1.5 K, suggesting that the ground state of AmO is very sensitive to disorder. We have also confirmed the loss of
is very sensitive to disorder. We have also confirmed the loss of  O NMR signal intensity over a wide temperature range below
O NMR signal intensity over a wide temperature range below 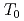 , and more than half of oxygen nuclei are undetectable at 1.5 K. This behavior reveals the persistence of slow and distributed spin fluctuations down to temperatures well below
, and more than half of oxygen nuclei are undetectable at 1.5 K. This behavior reveals the persistence of slow and distributed spin fluctuations down to temperatures well below 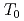 . In the paramagnetic state, strong NMR line broadening and spatially inhomogeneous spin fluctuations have been observed. The results are all indicative of short-range, spin-glass-like character for the magnetic transition in this system.
. In the paramagnetic state, strong NMR line broadening and spatially inhomogeneous spin fluctuations have been observed. The results are all indicative of short-range, spin-glass-like character for the magnetic transition in this system.
Tokunaga, Yo; Sakai, Hironori; Kambe, Shinsaku; Chudo, Hiroyuki; Osaka, Masahiko; Miwa, Shuhei; Nishi, Tsuyoshi; Nakada, Masami; Ito, Akinori; Homma, Yoshiya*
Materials Research Society Symposium Proceedings, Vol.1444, p.149 - 158, 2012/00
Times Cited Count:0 Percentile:0.11(Materials Science, Multidisciplinary)Besides the importance of AnO series (An; U, Np, Pu, Am) as a nuclear fuel, the magnetic properties of these compounds at low temperatures are particularly interesting. Their surprisingly varied physical properties continue to be of interest for both theory and experiment. In this study, we have performed NMR studies for the series of actinide dioxides. On the basis of
series (An; U, Np, Pu, Am) as a nuclear fuel, the magnetic properties of these compounds at low temperatures are particularly interesting. Their surprisingly varied physical properties continue to be of interest for both theory and experiment. In this study, we have performed NMR studies for the series of actinide dioxides. On the basis of  O-NMR studies, exotic magnetic orderings associated with multipole degrees of freedom on 5
O-NMR studies, exotic magnetic orderings associated with multipole degrees of freedom on 5 electrons have been identified in UO
electrons have been identified in UO (dipolar + quadrupolar ordering) and NpO
(dipolar + quadrupolar ordering) and NpO (octupolar + quadrupolar ordering), in contrast with the non-magnetic ground state of PuO
(octupolar + quadrupolar ordering), in contrast with the non-magnetic ground state of PuO . In AmO
. In AmO , our
, our  O-NMR data provide the first microscopic evidence for a phase transition as a bulk property in this system.
O-NMR data provide the first microscopic evidence for a phase transition as a bulk property in this system.
 and AmO
and AmO
Nishi, Tsuyoshi; Ito, Akinori*; Ichise, Kenichi; Arai, Yasuo
Journal of Nuclear Materials, 414(2), p.109 - 113, 2011/07
Times Cited Count:17 Percentile:77.43(Materials Science, Multidisciplinary)The thermal diffusivities of AmO and AmO
and AmO were measured using a laser flash method. The heat capacities of AmO
were measured using a laser flash method. The heat capacities of AmO and AmO
and AmO were measured using a drop calorimetry. The thermal conductivity was determined from the measured thermal diffusivity, heat capacity and bulk density. In these results, the heat capacity of AmO
were measured using a drop calorimetry. The thermal conductivity was determined from the measured thermal diffusivity, heat capacity and bulk density. In these results, the heat capacity of AmO was larger than that of AmO
was larger than that of AmO and close to those of UO
and close to those of UO and NpO
and NpO . The thermal conductivities of AmO
. The thermal conductivities of AmO and AmO
and AmO were found to decrease with increasing temperature in the temperature range investigated. The thermal conductivity of AmO
were found to decrease with increasing temperature in the temperature range investigated. The thermal conductivity of AmO from 473 to 773 K was slightly smaller than those of UO
from 473 to 773 K was slightly smaller than those of UO and PuO
and PuO and close to that of NpO
and close to that of NpO . On the other hand, the thermal conductivity of AmO
. On the other hand, the thermal conductivity of AmO with A-type rare earth oxide structure was smaller than that of AmO
with A-type rare earth oxide structure was smaller than that of AmO with fluorite structure and larger than that of non-stoichiometric AmO
with fluorite structure and larger than that of non-stoichiometric AmO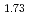 .
.
 O
O and AmO
and AmO with XAFS spectroscopy
with XAFS spectroscopyNishi, Tsuyoshi; Nakada, Masami; Suzuki, Chikashi; Shibata, Hiroki; Ito, Akinori; Akabori, Mitsuo; Hirata, Masaru
Journal of Nuclear Materials, 401(1-3), p.138 - 142, 2010/06
Times Cited Count:28 Percentile:86.66(Materials Science, Multidisciplinary)XAFS studies were performed in a study of americium sesquioxide (Am O
O ) with A-type rare earth oxide structure and americium dioxide (AmO
) with A-type rare earth oxide structure and americium dioxide (AmO ) with fluorite structure. EXAFS results for Am-L
) with fluorite structure. EXAFS results for Am-L absorption edge of Am
absorption edge of Am O
O and AmO
and AmO were good agreement with the crystallographic data from X-ray diffraction analysis. In order to characterize XANES in aspect of the electronic states, the theoretical assignments for the Am
were good agreement with the crystallographic data from X-ray diffraction analysis. In order to characterize XANES in aspect of the electronic states, the theoretical assignments for the Am O
O and AmO
and AmO were performed with the all-electron full potential linearized augmented plane wave (FP-LAPW) method. The theoretical XANES spectra of Am
were performed with the all-electron full potential linearized augmented plane wave (FP-LAPW) method. The theoretical XANES spectra of Am O
O and AmO
and AmO well reproduced the experimental ones. In addition, it was found that the white line peak was created due to the interaction between Am-d and O-p components, and the broad peak and the tail peak were created due to the interaction between Am-d and O-d component.
well reproduced the experimental ones. In addition, it was found that the white line peak was created due to the interaction between Am-d and O-p components, and the broad peak and the tail peak were created due to the interaction between Am-d and O-d component.
Nishi, Tsuyoshi; Takano, Masahide; Ito, Akinori; Miyata, Seiichi; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
IOP Conference Series; Materials Science and Engineering, 9, p.012017_1 - 012017_8, 2010/05
Times Cited Count:2 Percentile:70.97(Chemistry, Inorganic & Nuclear)The thermal diffusivities and heat capacities of transuranium nitride solid solutions, (Np,Am)N and (Pu,Am)N, were measured by using a laser flash method and a drop calorimetry, respectively. The thermal conductivities of these samples were determined from the measured thermal diffusivities, heat capacities and bulk densities. The thermal conductivities of (Np,Am)N and (Pu,Am)N increased with temperature over the temperature range investigated. The increases in the thermal conductivities were probably due to the increase of electrical components. In addition, the thermal conductivities of (Np,Am)N and (Pu,Am)N decreased with increasing Am contents. It could be considered that the decreases in the thermal conductivities correspond to the lowering of electronic contribution.
Tokunaga, Yo; Nishi, Tsuyoshi; Kambe, Shinsaku; Nakada, Masami; Ito, Akinori; Homma, Yoshiya*; Sakai, Hironori; Chudo, Hiroyuki
Journal of the Physical Society of Japan, 79(5), p.053705_1 - 053705_4, 2010/05
Times Cited Count:15 Percentile:64.85(Physics, Multidisciplinary)We report here the first NMR study of americium dioxide (AmO ). More than 30 years ago, a phase transition was suggested to occur in this compound at 8.5 K based on magnetic susceptibility data, while no evidence had been obtained from microscopic measurements. We have prepared a powder sample of
). More than 30 years ago, a phase transition was suggested to occur in this compound at 8.5 K based on magnetic susceptibility data, while no evidence had been obtained from microscopic measurements. We have prepared a powder sample of  AmO
AmO containing 90 at.%
containing 90 at.%  O and have performed
O and have performed  O NMR at temperatures ranging from 1.5 K to 200 K. After a sudden drop of the
O NMR at temperatures ranging from 1.5 K to 200 K. After a sudden drop of the  O NMR signal intensity below 8.5 K, at 1.5 K we have observed an extremely broad spectrum covering a range of
O NMR signal intensity below 8.5 K, at 1.5 K we have observed an extremely broad spectrum covering a range of  14 kOe in applied field. These data provide the first microscopic evidence for a phase transition as a bulk property in this system. In addition, the
14 kOe in applied field. These data provide the first microscopic evidence for a phase transition as a bulk property in this system. In addition, the  O NMR spectrum has been found to split into two peaks in the paramagnetic state, an effect which has not been reported for actinide dioxides studied up to now. We suggest that the splitting is induced by self-radiation damage from the alpha decay of
O NMR spectrum has been found to split into two peaks in the paramagnetic state, an effect which has not been reported for actinide dioxides studied up to now. We suggest that the splitting is induced by self-radiation damage from the alpha decay of  Am.
Am.
Nishi, Tsuyoshi; Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), 8 Pages, 2010/00
Neptunium nitride (NpN) and americium nitride (AmN) were prepared by carbothermic reduction of the respective dioxides. The thermal diffusivities of NpN and AmN were measured by using a laser flash method. The heat capacities of NpN and AmN were determined with the drop calorimetry. The thermal diffusivity of NpN tends to remain unchanged with increasing temperature in the temperature range from 473 to 1473 K, and that of AmN tends to slightly decrease with increasing temperature in the same temperature range. The heat capacity of NpN obtained was close to those of UN and PuN, while that of AmN was slightly smaller than those of UN, NpN and PuN. The thermal conductivities of NpN and AmN were determined from the measured thermal diffusivities, heat capacities and bulk densities. It was found that the thermal conductivities of NpN and AmN slightly increased with temperature in the temperature range investigated.
Nishi, Tsuyoshi; Ito, Akinori; Takano, Masahide; Numata, Masami; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Journal of Nuclear Materials, 377(3), p.467 - 469, 2008/07
Times Cited Count:12 Percentile:61.74(Materials Science, Multidisciplinary)The specific heat capacities of NpN and AmN were determined by the drop calorimetry method. The NpN and AmN samples were prepared by the carbothermic reduction of the respective dioxides. The specific heat capacity of NpN obtained was in good agreement with the reported values in the temperature range from 334 to 1067 K, which was close to those of UN and PuN. The specific heat capacity of AmN was obtained experimentally for the first time, which was slightly smaller than those of UN, NpN and PuN in the temperature range from 354 to 1071 K.
Nishi, Tsuyoshi; Ito, Akinori; Takano, Masahide; Numata, Masami; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Journal of Nuclear Materials, 376(1), p.78 - 82, 2008/05
Times Cited Count:33 Percentile:88.13(Materials Science, Multidisciplinary)The thermal diffusivity of neptunium dioxide was measured in the temperature range from 473 to 1473 K by a laser flash method. The thermal diffusivity slightly decreased with increasing temperature in the temperature range investigated. The specific heat capacity of NpO was measured in the temperature range from 334 to 1071 K by a drop calorimetry method. The specific heat capacity of NpO
was measured in the temperature range from 334 to 1071 K by a drop calorimetry method. The specific heat capacity of NpO determined in this study was slightly larger than that of UO
determined in this study was slightly larger than that of UO and about 7 % smaller than that of PuO
and about 7 % smaller than that of PuO . The thermal conductivity of NpO
. The thermal conductivity of NpO was determined from the thermal diffusivity, the specific heat capacity and the bulk density. It was found that the thermal conductivity of NpO
was determined from the thermal diffusivity, the specific heat capacity and the bulk density. It was found that the thermal conductivity of NpO from 873 to 1473 K existed between those of UO
from 873 to 1473 K existed between those of UO and PuO
and PuO .
.
Nishi, Tsuyoshi; Ito, Akinori; Takano, Masahide; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of 3rd International ATALANTE Conference (ATALANTE 2008) (CD-ROM), 6 Pages, 2008/05
The thermal diffusivities of americium oxide and neptunium dioxide were determined by a laser flash method. It was found that the thermal diffusivities of AmO and NpO
and NpO decreased with increasing temperature. It was also found that the decrease in O/Am ratio during the thermal diffusivity measurements under vacuum resulted in a slight decrease in thermal diffusivity of AmO
decreased with increasing temperature. It was also found that the decrease in O/Am ratio during the thermal diffusivity measurements under vacuum resulted in a slight decrease in thermal diffusivity of AmO . The thermal conductivities of AmO
. The thermal conductivities of AmO and NpO
and NpO were evaluated from the measured thermal diffusivities, heat capacities and bulk densities. The thermal conductivity of AmO
were evaluated from the measured thermal diffusivities, heat capacities and bulk densities. The thermal conductivity of AmO was smaller than those of the literature values of UO
was smaller than those of the literature values of UO and PuO
and PuO . On the other hand, the thermal conductivity of NpO
. On the other hand, the thermal conductivity of NpO from 873 to 1473 K lay between those of UO
from 873 to 1473 K lay between those of UO and PuO
and PuO . The thermal conductivities of AmO
. The thermal conductivities of AmO and NpO
and NpO decreased with increasing temperature in the temperature range investigated.
decreased with increasing temperature in the temperature range investigated.
Nishi, Tsuyoshi; Nakada, Masami; Ito, Akinori; Suzuki, Chikashi; Hirata, Masaru; Akabori, Mitsuo
Journal of Nuclear Materials, 374(3), p.339 - 343, 2008/03
Times Cited Count:20 Percentile:77.66(Materials Science, Multidisciplinary)EXAFS and XANES analysis were applied in a study of americium dioxide (AmO ) with fluorite structure. EXAFS result for Am-L
) with fluorite structure. EXAFS result for Am-L absorption edge of AmO
absorption edge of AmO was good agreement with the long-ranged structural data from X-ray diffraction analysis. In order to characterize XANES in aspect of the electronic structure, the theoretical assignment for the AmO
was good agreement with the long-ranged structural data from X-ray diffraction analysis. In order to characterize XANES in aspect of the electronic structure, the theoretical assignment for the AmO was performed with the relativistic DV-X
was performed with the relativistic DV-X molecular orbital method. The calculated XANES spectrum well reproduced the experimental spectrum. The theoretical assignment of the XANES spectra is very useful for the development of MA-MOX fuel for the future nuclear fuel cycle.
molecular orbital method. The calculated XANES spectrum well reproduced the experimental spectrum. The theoretical assignment of the XANES spectra is very useful for the development of MA-MOX fuel for the future nuclear fuel cycle.

Nishi, Tsuyoshi; Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo; Numata, Masami
Journal of Nuclear Materials, 373(1-3), p.295 - 298, 2008/02
Times Cited Count:16 Percentile:70.82(Materials Science, Multidisciplinary)The thermal diffusivity of americium oxide was determined in the temperature range from 299 to 1473 K by a laser flash method. The thermal diffusivity of AmO decreased with increasing temperature. The thermal conductivity of AmO
decreased with increasing temperature. The thermal conductivity of AmO was estimated from the measured thermal diffusivity, the specific heat capacity and the bulk density. It was found that the thermal conductivity of AmO
was estimated from the measured thermal diffusivity, the specific heat capacity and the bulk density. It was found that the thermal conductivity of AmO decreased with increasing temperature over the temperature range investigated. It was also found that the decrease in O/Am ratio during the thermal diffusivity measurements under vacuum resulted in a slight decrease in thermal conductivity of AmO
decreased with increasing temperature over the temperature range investigated. It was also found that the decrease in O/Am ratio during the thermal diffusivity measurements under vacuum resulted in a slight decrease in thermal conductivity of AmO .
.
Nishi, Tsuyoshi; Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Minato, Kazuo; Kizaki, Minoru
Journal of Nuclear Materials, 355(1-3), p.114 - 118, 2006/09
Times Cited Count:20 Percentile:78.83(Materials Science, Multidisciplinary)The thermal diffusivity of AmN was measured from 373 to 1473 K by a laser flash method. The AmN sample was prepared from AmO by the carbothermic reduction. The obtained AmN product was grinded and pressed at about 400 MPa to form a disk. The disk was sintered at 1823 K for 10 hours in flowing N
by the carbothermic reduction. The obtained AmN product was grinded and pressed at about 400 MPa to form a disk. The disk was sintered at 1823 K for 10 hours in flowing N +4%H
+4%H gas. The thermal diffusivity slightly decreased from 3.4
gas. The thermal diffusivity slightly decreased from 3.4 10
10 to 2.8
to 2.8 10
10 m
m /s with increasing temperature. As the specific heat capacity of AmN was not available in literature, the thermal conductivity of AmN was tentatively estimated from the measured thermal diffusivity, bulk density and the specific heat capacity of PuN. It was found that the thermal conductivity of AmN slightly increased with temperature. The thermal conductivity of AmN corrected to 100%TD was found to be smaller than those of UN, NpN and PuN, whereas that of AmN was larger than those of UO
/s with increasing temperature. As the specific heat capacity of AmN was not available in literature, the thermal conductivity of AmN was tentatively estimated from the measured thermal diffusivity, bulk density and the specific heat capacity of PuN. It was found that the thermal conductivity of AmN slightly increased with temperature. The thermal conductivity of AmN corrected to 100%TD was found to be smaller than those of UN, NpN and PuN, whereas that of AmN was larger than those of UO and (U
and (U Pu
Pu )O
)O .
.
Minato, Kazuo; Takano, Masahide; Nishi, Tsuyoshi; Ito, Akinori; Akabori, Mitsuo
Recent Advances in Actinide Science, p.317 - 322, 2006/06
To reduce the radiotoxicity of the high-level waste and to use the repository efficiently, recycling of minor actinides (MA: Np, Am, Cm) as well as plutonium is an option for the future nuclear fuel cycle. For MA-bearing fuel development, new facilities with inert atmosphere were installed and the thermal properties of minor actinide compounds, especially nitrides and oxides, were measured. Minor actinide nitrides were prepared by carbothermic reduction of the oxides. Lattice parameter and its thermal expansion were measured by high-temperature X-ray diffraction, and thermal diffusivity by laser flash method.
Nishi, Tsuyoshi; Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Minato, Kazuo; Kizaki, Minoru
JAERI-Tech 2005-051, 13 Pages, 2005/09
An apparatus has been developed to measure the thermal diffusivities of minor actinide (MA) compounds. By installing the laser flash apparatus in a glove box with highly purified inert gas atmosphere, the thermal diffusivities measurement of MA compounds of  -decay nuclides was enabled. A new sample holder has been also developed to measure the thermal diffusivities of very small samples. The performance of this new apparatus was confirmed by measuring the thermal diffusivities of small samples of tantalum, nickel and cerium oxides. The thermal diffusivity values obtained in this work agreed well with the literature values and the values measured by a conventional thermal diffusivities measurement apparatus. Accordingly, this apparatus was found to be useful for thermal diffusivities measurement of MA compounds.
-decay nuclides was enabled. A new sample holder has been also developed to measure the thermal diffusivities of very small samples. The performance of this new apparatus was confirmed by measuring the thermal diffusivities of small samples of tantalum, nickel and cerium oxides. The thermal diffusivity values obtained in this work agreed well with the literature values and the values measured by a conventional thermal diffusivities measurement apparatus. Accordingly, this apparatus was found to be useful for thermal diffusivities measurement of MA compounds.
Takeyama, Akinori; Yamamoto, Shunya; Ito, Hiroshi; Yoshikawa, Masahito
Nuclear Instruments and Methods in Physics Research B, 232(1-4), p.333 - 337, 2005/05
Times Cited Count:0 Percentile:0.01(Instruments & Instrumentation)Cu precipitates were formed on Si(100) by 200 keV Cu ion implantation and subsequent annealing at 773 K. The shape of the Cu precipitates evolved from a large rectangle to a small elongated pyramid with increasing annealing time. This shape evolution seemed to result from the epitaxial formation of Cu precipitates to minimize the interfacial energy between the precipitate and the Cu implanted substrate. The average density of Cu precipitates monotonously increased and the average diameter of Cu precipitates decreased with increasing annealing time up to 1 h. These indicate that the morphology, size and average density of Cu precipitates can be controlled by varying annealing time, and that Cu ion implantation and subsequent annealing were effective in producing a substrate dispersed with catalytic particles for oxide nanorods growth.
Takeyama, Akinori; Yamamoto, Shunya; Yoshikawa, Masahito; Ito, Hiroshi
Japanese Journal of Applied Physics, Part 1, 44(1B), p.750 - 753, 2005/01
Times Cited Count:0 Percentile:0(Physics, Applied)Pyramid shaped Cu precipitates were formed on Si (100) surface as a result of 200 keV Cu ion implantation and subsequent annealing. Then, ZnO nanorods were successfully synthesized on the Cu implanted substrates by chemical vapor transport (CVT). Hexagonal shaped nanorods with a diameter of 200 nm were grown nearly perpendicular to the Cu implanted substrate and their average density was increased as increasing that of Cu precipitates. The facts strongly indicate the Cu precipitates served as the catalytic particles for the growth of ZnO rods.
Minato, Kazuo; Akabori, Mitsuo; Takano, Masahide; Arai, Yasuo; Nakajima, Kunihisa; Ito, Akinori; Ogawa, Toru
Journal of Nuclear Materials, 320(1-2), p.18 - 24, 2003/09
Times Cited Count:53 Percentile:94.59(Materials Science, Multidisciplinary)In the Japan Atomic Energy Research Institute, the concept of the transmutation of minor actinides (MA: Np, Am and Cm) with accelerator-driven systems is being studied. The MA nitride fuel has been chosen as a candidate because of the possible mutual solubility among the actinide mononitrides and excellent thermal properties besides supporting hard neutron spectrum. MA nitrides of AmN, (Am,Y)N, (Am,Zr)N and (Cm0.4Pu0.6)N were prepared from the oxides by the carbothermic reduction method. The prepared MA nitrides were examined by X-ray diffraction and the contents of impurities of oxygen and carbon were measured. The fabrication conditions for MA nitrides were improved so as to reduce the impurity contents. For an irradiation test of U-free nitride fuels, pellets of (Pu,Zr)N and PuN+TiN were prepared and a He-bonded fuel pin was fabricated. The irradiation test started in May 2002 and will go on for two years in the Japan Materials Testing Reactor.
Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Minato, Kazuo; Numata, Masami
Proceedings of GLOBAL2003 Atoms for Prosperity; Updating Eisenhower's Global Vision for Nuclear Energy (CD-ROM), p.2285 - 2291, 2003/00
Stability of AmN and (Am,Zr)N was studied comparatively from the viewpoints of the hydrolytic and evaporative behavior. AmN powder reacted with moisture to form hydroxide Am(OH) , while the solid solution (Am
, while the solid solution (Am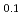 Zr
Zr )N remained stable as long as 1000 hours. Stabilization effect of ZrN was found to depend significantly on its mole fraction from the experiments on (Dy,Zr)N. In the oxidation experiments on (Dy,Zr)N by TG-DTA technique, rapid weight gain by the oxidation occurred above 700 K. Effect of ZrN on the stability against oxygen was slight. Nitrogen release by the evaporation of AmN and (Am
)N remained stable as long as 1000 hours. Stabilization effect of ZrN was found to depend significantly on its mole fraction from the experiments on (Dy,Zr)N. In the oxidation experiments on (Dy,Zr)N by TG-DTA technique, rapid weight gain by the oxidation occurred above 700 K. Effect of ZrN on the stability against oxygen was slight. Nitrogen release by the evaporation of AmN and (Am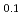 Zr
Zr )N in He gas flow was measured by gas chromatography. Evaporation rate constants of AmN were obtained at 1623-1733 K. Although the evaporation rate constant of AmN in the solid solution were lower than those of the pure AmN, the selective evaporation of AmN from the solid slution were recognized, which resulted in a decrease in the Am mole fraction.
)N in He gas flow was measured by gas chromatography. Evaporation rate constants of AmN were obtained at 1623-1733 K. Although the evaporation rate constant of AmN in the solid solution were lower than those of the pure AmN, the selective evaporation of AmN from the solid slution were recognized, which resulted in a decrease in the Am mole fraction.
Okamoto, Yoshihiro; Akabori, Mitsuo; Ito, Akinori; Ogawa, Toru
Journal of Nuclear Science and Technology, 39(Suppl.3), p.638 - 641, 2002/11
We report local structural features of molten UCl with LiCl-KCl eutectic probed by the U L
with LiCl-KCl eutectic probed by the U L -edge XAFS(X-ray absorption fine structure). The XAFS measurements were performed in a transmission mode at the BL27B station of the Photon Factory(High Energy Accelerator Organization, Tsukuba, JAPAN). Sample prepared by chlorination of uranium hydride and then reduction with zinc powder was sealed in a quartz cell under reduced pressure. The nearest U
-edge XAFS(X-ray absorption fine structure). The XAFS measurements were performed in a transmission mode at the BL27B station of the Photon Factory(High Energy Accelerator Organization, Tsukuba, JAPAN). Sample prepared by chlorination of uranium hydride and then reduction with zinc powder was sealed in a quartz cell under reduced pressure. The nearest U -Cl
-Cl distance and the coordination number of Cl
distance and the coordination number of Cl around U
around U ion were obtained by a curve fitting of the 1st shell XAFS function k
ion were obtained by a curve fitting of the 1st shell XAFS function k
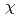 (k). The pair potential in the U
(k). The pair potential in the U -Cl
-Cl correlation was evaluated from XAFS simulation by combinational use of the MD and the FEFF8. In addition, valence state of uranium in the melt was evaluated by XANES(X-ray absorption near edge structure) spectra.
correlation was evaluated from XAFS simulation by combinational use of the MD and the FEFF8. In addition, valence state of uranium in the melt was evaluated by XANES(X-ray absorption near edge structure) spectra.