Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:21 Percentile:79.8(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
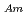 ) and Cm (
) and Cm (
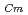 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
Tsutsui, Nao; Ban, Yasutoshi; Ishii, Sho*; Ito, Sayumi*; Suzuki, Hideya; Matsumura, Tatsuro; Inaba, Yusuke*; Takeshita, Kenji*
no journal, ,
no abstracts in English
Tsutsui, Nao; Nakase, Masahiko*; Ito, Sayumi*; Matsumura, Tatsuro; Takeshita, Kenji*
no journal, ,
To separate minor acitinides from lanthanides in nitric acid, we focused on 1,10-phenanthroline-2,9-diamides (PTDAs) which consist of both soft and hard donor atoms. The present study employed a PTDA having dodecyl groups in N atoms of amides groups, TDdPTDA, and its extraction property toward Am, Cm, and Eu was studied.
Ito, Sayumi*; Tsutsui, Nao; Nakase, Masahiko*; Matsumura, Tatsuro; Takeshita, Kenji*
no journal, ,
We focused on 1,10-phenanthroline-2,9-diamides (PTDAs) for mutual separation of minor actinides (MAs) from lanthanides (Lns). PTDAs are hybrid-type ligands which consist of both soft N and hard O donors and show high extraction selectivity for MAs over Lns. They also have the symmetrical structures, and thus they are easy to synthesize. Furthermore, PTDAs have little detrimental effect on environment when they are incinerated. In this study, we synthesized novel PTDAs having phenyl groups and alkyl groups on the amide groups. The effectiveness of the novel PTDAs was evaluated from the viewpoint of extraction and separation properties of Lns and MAs.
Ito, Sayumi*; Nakase, Masahiko*; Tsutsui, Nao; Matsumura, Tatsuro; Takeshita, Kenji*
no journal, ,
no abstracts in English
Tsutsui, Nao; Nakase, Masahiko*; Ito, Sayumi*; Ban, Yasutoshi; Matsumura, Tatsuro; Takeshita, Kenji*
no journal, ,
The extraction properties of three 1,10-phenanthroline-2,9-diamide derivatives (
 ,
,
 -diethly-
-diethly-
 ,
,
 -bis(4-ethylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Et(EtPh)PTDA),
-bis(4-ethylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Et(EtPh)PTDA), 
 ,
,
 -dihexyl-
-dihexyl-
 ,
, 
 -bis(4-hexylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Hex(HexPh)PTDA), and
-bis(4-hexylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Hex(HexPh)PTDA), and 
 ,
, 
 -didodecyl-
-didodecyl-
 ,
, 
 -bis(4-dodecylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Dd(DdPh)PTDA)) were studied for the separation of minor actinides (Am and Cm) and lanthanide (Eu) using single-stage batch methods. The results demonstrated that the PTDA derivatives extracted Am more efficiently than Eu or Cm. Furthermore, PTDA functionalized with short alkyl chains exhibited higher distribution ratios toward Eu, Am, and Cm when compared with those exhibited with longer chains. It was also revealed that the separation factor of Am with respect to Eu was relatively large, and that the alkyl chain length did not affect the separation factor when the same concentration of PTDA was used.
-bis(4-dodecylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Dd(DdPh)PTDA)) were studied for the separation of minor actinides (Am and Cm) and lanthanide (Eu) using single-stage batch methods. The results demonstrated that the PTDA derivatives extracted Am more efficiently than Eu or Cm. Furthermore, PTDA functionalized with short alkyl chains exhibited higher distribution ratios toward Eu, Am, and Cm when compared with those exhibited with longer chains. It was also revealed that the separation factor of Am with respect to Eu was relatively large, and that the alkyl chain length did not affect the separation factor when the same concentration of PTDA was used.