Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakahara, Masaumi; Watanabe, So; Kimura, Shuya; Sasaki, Misa*; Inagaki, Hiromitsu*; Moriguchi, Tetsuji*
Progress in Nuclear Energy, 172, p.105195_1 - 105195_8, 2024/07
A novel removal technique with ultrafine bubbles has been proposed for decommissioning of nuclear facilities. The performance of removal technology with ultrafine bubbles was evaluated in the removal experiments with non-radioactive materials, simulated contaminants precipitated Co oxides. To investigate the influence of difference in the chemical forms, the decontamination experiments were carried out with the fuel pin end plugs contaminated radioactive materials in a hot cell.
 Be and
Be and 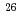 Al under direct muon-induced spallation in granite quartz and its implications for past high-energy cosmic ray fluxes
Al under direct muon-induced spallation in granite quartz and its implications for past high-energy cosmic ray fluxesSakurai, Hirohisa*; Kurebayashi, Yutaka*; Suzuki, Soichiro*; Horiuchi, Kazuho*; Takahashi, Yui*; Doshita, Norihiro*; Kikuchi, Satoshi*; Tokanai, Fuyuki*; Iwata, Naoyoshi*; Tajima, Yasushi*; et al.
Physical Review D, 109(10), p.102005_1 - 102005_18, 2024/05
Secular variations of galactic cosmic rays (GCRs) are inseparably associated with the galactic activities and should reflect the environments of the local galactic magnetic field, interstellar clouds, and nearby supernova remnants. The high-energy muons produced in the atmosphere by high-energy GCRs can penetrate deep underground and generate radioisotopes in the rock. As long lived radionuclides such as  Be and
Be and 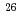 Al have been accumulating in these rocks, concentrations of
Al have been accumulating in these rocks, concentrations of  Be and
Be and 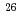 Al can be used to estimate the long-term variations in high-energy muon yields, corresponding to those in the high-energy GCRs over a few million years. This study measured the production cross sections for muon induced
Al can be used to estimate the long-term variations in high-energy muon yields, corresponding to those in the high-energy GCRs over a few million years. This study measured the production cross sections for muon induced  Be and
Be and 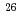 Al by irradiating positive muons with the momentum of 160 GeV/c on the synthetic silica plates and the granite core at the COMPASS experiment line in CERN SPS. In addition, it the contributions of the direct muon spallation reaction and the nuclear reactions by muon-induced particles on the production of long lived radionuclides in the rocks were clarified.
Al by irradiating positive muons with the momentum of 160 GeV/c on the synthetic silica plates and the granite core at the COMPASS experiment line in CERN SPS. In addition, it the contributions of the direct muon spallation reaction and the nuclear reactions by muon-induced particles on the production of long lived radionuclides in the rocks were clarified.
Tonna, Ryutaro*; Sasaki, Takayuki*; Okamoto, Yoshihiro; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*
Journal of Nuclear Materials, 589, p.154862_1 - 154862_10, 2024/02
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)The dissolution behavior of FeUO compounds formed by a high-temperature reaction of UO
compounds formed by a high-temperature reaction of UO with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U
with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U O
O and Fe
and Fe O
O as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO
as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO
compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U
dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U O
O ).
).
Sato, Nobuaki*; Kirishima, Akira*; Sasaki, Takayuki*; Takano, Masahide; Kumagai, Yuta; Sato, Soichi; Tanaka, Kosuke
Current Location of Fuel Debris Chemistry, 178 Pages, 2023/11
Considerable efforts have been devoted to the decommissioning of the TEPCO's Fukushima Daiichi Nuclear Power Station (1F) and now the retrieval of fuel debris is being proceeded on a trial basis. It can be said that the succession of science and technology related to debris, that is, human resource development, is important and indispensable. For that reason, we thought that a specific textbook on decommissioning is necessary. Regarding the 1F fuel debris, we still do not know enough, and it would be difficult to describe the details. However, 12 years have passed since the accident, and we have come to understand the situation of 1F to a certain extent. At this stage, it is essential for future development to organize the current situation by combining examples of past severe accidents. Therefore, we presented in this book the current state of fuel debris chemistry research from the perspectives of solid chemistry, solution chemistry, analytical chemistry, radiochemistry, and radiation chemistry.
Kusaka, Ryoji; Kumagai, Yuta; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 60(5), p.603 - 613, 2023/05
Times Cited Count:1 Percentile:29.26(Nuclear Science & Technology) , Zr, and stainless steel and leaching behavior of the fission products and matrix elements
, Zr, and stainless steel and leaching behavior of the fission products and matrix elementsTonna, Ryutaro*; Sasaki, Takayuki*; Kodama, Yuji*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta; Kusaka, Ryoji; Watanabe, Masayuki
Nuclear Engineering and Technology, 55(4), p.1300 - 1309, 2023/04
Times Cited Count:2 Percentile:68.31(Nuclear Science & Technology)Simulated debris was synthesized using UO , Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO
, Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO , whereas a (U,Zr)O
, whereas a (U,Zr)O solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U
solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U O
O and (Fe,Cr)UO
and (Fe,Cr)UO phases formed at 1473 K whereas a (U,Zr)O
phases formed at 1473 K whereas a (U,Zr)O solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
 , Zr, and stainless-steel
, Zr, and stainless-steelKirishima, Akira*; Akiyama, Daisuke*; Kumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Sasaki, Takayuki*; Sato, Nobuaki*
Journal of Nuclear Materials, 567, p.153842_1 - 153842_15, 2022/08
Times Cited Count:5 Percentile:76.47(Materials Science, Multidisciplinary)To understand the chemical structure and stability of nuclear fuel debris consisting of UO , Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO
, Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO -SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600
-SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600 C, in inert (Ar) or oxidative (Ar + 2% O
C, in inert (Ar) or oxidative (Ar + 2% O ) atmospheres.
) atmospheres.  Np and
Np and  Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M
Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M ssbauer spectroscopy, which provided the major uranium phase of the UO
ssbauer spectroscopy, which provided the major uranium phase of the UO  -SUS-Zr debris was the solid solution of U
-SUS-Zr debris was the solid solution of U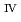 O
O (s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO
(s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO -SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
-SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
 O
O solution
solutionKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Journal of Nuclear Science and Technology, 59(8), p.961 - 971, 2022/08
Times Cited Count:2 Percentile:50.96(Nuclear Science & Technology)We investigated potential degradation of fuel debris caused by H O
O , which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H
, which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H O
O solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H
solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H O
O . Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H
. Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H O
O reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO
reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO improves the stability of fuel debris against H
improves the stability of fuel debris against H O
O reaction.
reaction.
Ohshima, Hiroyuki; Morishita, Masaki*; Aizawa, Kosuke; Ando, Masanori; Ashida, Takashi; Chikazawa, Yoshitaka; Doda, Norihiro; Enuma, Yasuhiro; Ezure, Toshiki; Fukano, Yoshitaka; et al.
Sodium-cooled Fast Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.3, 631 Pages, 2022/07
This book is a collection of the past experience of design, construction, and operation of two reactors, the latest knowledge and technology for SFR designs, and the future prospects of SFR development in Japan. It is intended to provide the perspective and the relevant knowledge to enable readers to become more familiar with SFR technology.
 O
O -induced oxidative degradation of simulated fuel debris
-induced oxidative degradation of simulated fuel debrisKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Hoshasen Kagaku (Internet), (113), p.61 - 64, 2022/04
The severe accident at TEPCO's Fukushima Daiichi Nuclear Power Station resulted in generation of fuel debris. The fuel debris is in contact with water and the radiolysis of water can accelerate degradation of the debris. The analysis of particles sampled from inside or near the damaged reactors indicates the complicated compositions of the fuel debris. It is challenging to estimate the effect of water radiolysis on such a complicated material. Therefore, in this study, we investigated the potential degradation process by leaching experiments of simulated fuel debris in aqueous H O
O solution. The results show that the reaction of H
solution. The results show that the reaction of H O
O induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H
induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H O
O . These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
. These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki; Sasaki, Takayuki*; Uehara, Akihiro*; Takeda, Shino*; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Kobayashi, Taishi*
The Chemistry of Thorium, Plutonium and MA, 254 Pages, 2022/03
The chemistry of nuclear materials such as Thorium (Part 1) and Plutonium (Part 2) was described in relation from the fundamentals on solid chemistry and solution chemistry to the practicals on the experiment and evaluation method in detail. Minor actinides such as Neptunium, Americium, Curium and Protoactinium, was introduced the basics on the solid and solution chemistry.
 O
O ; Application of Raman imaging technique to uranium compound
; Application of Raman imaging technique to uranium compoundKusaka, Ryoji; Kumagai, Yuta; Yomogida, Takumi; Takano, Masahide; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 58(6), p.629 - 634, 2021/06
Times Cited Count:7 Percentile:65.65(Nuclear Science & Technology)Takahashi, Atsushi*; Chiba, Mirei*; Tanahara, Akira*; Aida, Jun*; Shimizu, Yoshinaka*; Suzuki, Toshihiko*; Murakami, Shinobu*; Koarai, Kazuma; Ono, Takumi*; Oka, Toshitaka; et al.
Scientific Reports (Internet), 11(1), p.10355_1 - 10355_11, 2021/05
Times Cited Count:6 Percentile:40.04(Multidisciplinary Sciences)Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki; Sasaki, Takayuki*; Uehara, Akihiro*; Takeda, Shino*
Uran No Kagaku (II); Hoho To Jissen, 143 Pages, 2021/03
This book describes necessary facts when readers would have an opportunity to treat Uranium for experiments. In the content, the method section shows experimental facilities and equipment including method, and the practical section mentions solution and solid state experiments using Uranium and/or radioisotopes.
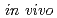 dosimetry system based on an optical fiber-coupled microsized photostimulable phosphor for stereotactic body radiation therapy
dosimetry system based on an optical fiber-coupled microsized photostimulable phosphor for stereotactic body radiation therapyYada, Ryuichi*; Maenaka, Kazusuke*; Miyamoto, Shuji*; Okada, Go*; Sasakura, Aki*; Ashida, Motoi*; Adachi, Masashi*; Sato, Tatsuhiko; Wang, T.*; Akasaka, Hiroaki*; et al.
Medical Physics, 47(10), p.5235 - 5249, 2020/10
Times Cited Count:7 Percentile:51.1(Radiology, Nuclear Medicine & Medical Imaging)The 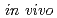 dosimeter system is capable of real-time, accurate, and precise measurement under stereotactic body radiation therapy (SBRT) conditions. The probe is smaller than a conventional dosimeter, has excellent spatial resolution, and can be valuable in SBRT with a steep dose distribution over a small field. The developed PSP dosimeter system appears to be suitable for in vivo SBRT dosimetry.
dosimeter system is capable of real-time, accurate, and precise measurement under stereotactic body radiation therapy (SBRT) conditions. The probe is smaller than a conventional dosimeter, has excellent spatial resolution, and can be valuable in SBRT with a steep dose distribution over a small field. The developed PSP dosimeter system appears to be suitable for in vivo SBRT dosimetry.
Strasser, P.*; Abe, Mitsushi*; Aoki, Masaharu*; Choi, S.*; Fukao, Yoshinori*; Higashi, Yoshitaka*; Higuchi, Takashi*; Iinuma, Hiromi*; Ikedo, Yutaka*; Ishida, Katsuhiko*; et al.
EPJ Web of Conferences, 198, p.00003_1 - 00003_8, 2019/01
Times Cited Count:13 Percentile:98.93(Quantum Science & Technology)Ueno, Yasuhiro*; Aoki, Masaharu*; Fukao, Yoshinori*; Higashi, Yoshitaka*; Higuchi, Takashi*; Iinuma, Hiromi*; Ikedo, Yutaka*; Ishida, Katsuhiko*; Ito, Takashi; Iwasaki, Masahiko*; et al.
Hyperfine Interactions, 238(1), p.14_1 - 14_6, 2017/11
Times Cited Count:3 Percentile:86.37(Physics, Atomic, Molecular & Chemical)Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo*; Yoshizuka, Kazuharu*
Proceedings of 21st International Solvent Extraction Conference (ISEC 2017) (Internet), p.131 - 134, 2017/11
Three tridentate extractants including soft and hard donor has been developed and examined. The extractants are termed as 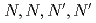 -tetraoctyl-diglycolamide (TODGA), methylimino-
-tetraoctyl-diglycolamide (TODGA), methylimino-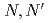 -dioctylacetamide (MIDOA) and
-dioctylacetamide (MIDOA) and 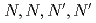 -tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
-tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
Kirishima, Akira*; Kuno, Atsushi*; Amamiya, Hiroki; Kubota, Takumi*; Kimuro, Shingo*; Amano, Yuki; Miyakawa, Kazuya; Iwatsuki, Teruki; Mizuno, Takashi; Sasaki, Takayuki*; et al.
Chemosphere, 168, p.798 - 806, 2017/02
Times Cited Count:3 Percentile:10.09(Environmental Sciences)For better understanding of the migration behavior of minor actinides (MA) in deep groundwater, the interaction of doped rare earth elements (REEs) and components in Horonobe deep groundwater was studied. Appx. 10 ppb of rare earth elements, i.e., Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Er, Tm and Yb were doped to the sample groundwater collected from a packed sections in borehole drilled from 140 m depth experiment drift of Horonobe underground research laboratory (URL), Hokkaido, Japan. Then, that groundwater was sequentially filtrated by 0.2 micron pore filter, 10 kDa, 3 kDa and 1 kDa of nominal molecular weight limit (NMWL) ultrafilters by keeping inert condition. After that, the filtrate solutions were analyzed by ICP-MS to determine the concentrations of retained REEs at each filtration steps, while the used filters were analyzed by the neutron activation analysis (NAA) and TOF-SIMS element mapping to know the amount and chemical speciation of trapped fraction of the REEs on each filter. A remarkable relation between the retention ratios of REEs in the filtrate solutions and the ionic radius was observed, i.e., smaller rare earth element solves more in liquid phase under the Horonobe groundwater condition. NAA and TOF-SIMS analyses revealed that certain portions of REEs were trapped by 0.2 micron pore filters as rare earth phosphates which corresponded with the predicted predominant species by a chemical equilibrium calculation for the Horonobe groundwater condition, while small portions of colloidal REEs were trapped by 10 kDa and 3 kDa NMWL ultrafilters. The result suggested that phosphate anion plays an important role in the chemical behavior of REEs in saline (seawater based) groundwater, which could be referred for the prediction of migration behavior of trivalent actinide released from the repository of radioactive waste in far future.
Strasser, P.*; Aoki, Masaharu*; Fukao, Yoshinori*; Higashi, Yoshitaka*; Higuchi, Takashi*; Iinuma, Hiromi*; Ikedo, Yutaka*; Ishida, Katsuhiko*; Ito, Takashi; Iwasaki, Masahiko*; et al.
Hyperfine Interactions, 237(1), p.124_1 - 124_9, 2016/12
Times Cited Count:7 Percentile:90.97(Physics, Atomic, Molecular & Chemical)