Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sato, Nobuaki*; Kirishima, Akira*; Sasaki, Takayuki*; Takano, Masahide; Kumagai, Yuta; Sato, Soichi; Tanaka, Kosuke
Current Location of Fuel Debris Chemistry, 178 Pages, 2023/11
Considerable efforts have been devoted to the decommissioning of the TEPCO's Fukushima Daiichi Nuclear Power Station (1F) and now the retrieval of fuel debris is being proceeded on a trial basis. It can be said that the succession of science and technology related to debris, that is, human resource development, is important and indispensable. For that reason, we thought that a specific textbook on decommissioning is necessary. Regarding the 1F fuel debris, we still do not know enough, and it would be difficult to describe the details. However, 12 years have passed since the accident, and we have come to understand the situation of 1F to a certain extent. At this stage, it is essential for future development to organize the current situation by combining examples of past severe accidents. Therefore, we presented in this book the current state of fuel debris chemistry research from the perspectives of solid chemistry, solution chemistry, analytical chemistry, radiochemistry, and radiation chemistry.
Kusaka, Ryoji; Kumagai, Yuta; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 60(5), p.603 - 613, 2023/05
Times Cited Count:1 Percentile:31.61(Nuclear Science & Technology) , Zr, and stainless steel and leaching behavior of the fission products and matrix elements
, Zr, and stainless steel and leaching behavior of the fission products and matrix elementsTonna, Ryutaro*; Sasaki, Takayuki*; Kodama, Yuji*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta; Kusaka, Ryoji; Watanabe, Masayuki
Nuclear Engineering and Technology, 55(4), p.1300 - 1309, 2023/04
Times Cited Count:1 Percentile:72.91(Nuclear Science & Technology)Simulated debris was synthesized using UO , Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO
, Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO , whereas a (U,Zr)O
, whereas a (U,Zr)O solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U
solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U O
O and (Fe,Cr)UO
and (Fe,Cr)UO phases formed at 1473 K whereas a (U,Zr)O
phases formed at 1473 K whereas a (U,Zr)O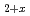 solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:3 Percentile:68.71(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe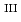 with increasing electron densities around Fe
with increasing electron densities around Fe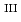 , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe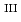
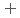 U
U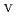
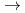 Fe
Fe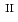
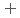 U
U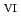 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr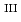 could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr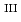 in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
 , Zr, and stainless-steel
, Zr, and stainless-steelKirishima, Akira*; Akiyama, Daisuke*; Kumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Sasaki, Takayuki*; Sato, Nobuaki*
Journal of Nuclear Materials, 567, p.153842_1 - 153842_15, 2022/08
Times Cited Count:4 Percentile:78.52(Materials Science, Multidisciplinary)To understand the chemical structure and stability of nuclear fuel debris consisting of UO , Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO
, Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO -SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600
-SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600 C, in inert (Ar) or oxidative (Ar + 2% O
C, in inert (Ar) or oxidative (Ar + 2% O ) atmospheres.
) atmospheres. 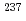 Np and
Np and 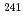 Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M
Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M ssbauer spectroscopy, which provided the major uranium phase of the UO
ssbauer spectroscopy, which provided the major uranium phase of the UO  -SUS-Zr debris was the solid solution of U
-SUS-Zr debris was the solid solution of U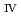 O
O (s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO
(s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO -SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
-SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
 O
O solution
solutionKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Journal of Nuclear Science and Technology, 59(8), p.961 - 971, 2022/08
Times Cited Count:2 Percentile:53.91(Nuclear Science & Technology)We investigated potential degradation of fuel debris caused by H O
O , which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H
, which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H O
O solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H
solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H O
O . Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H
. Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H O
O reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO
reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO improves the stability of fuel debris against H
improves the stability of fuel debris against H O
O reaction.
reaction.
 O
O -induced oxidative degradation of simulated fuel debris
-induced oxidative degradation of simulated fuel debrisKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Hoshasen Kagaku (Internet), (113), p.61 - 64, 2022/04
The severe accident at TEPCO's Fukushima Daiichi Nuclear Power Station resulted in generation of fuel debris. The fuel debris is in contact with water and the radiolysis of water can accelerate degradation of the debris. The analysis of particles sampled from inside or near the damaged reactors indicates the complicated compositions of the fuel debris. It is challenging to estimate the effect of water radiolysis on such a complicated material. Therefore, in this study, we investigated the potential degradation process by leaching experiments of simulated fuel debris in aqueous H O
O solution. The results show that the reaction of H
solution. The results show that the reaction of H O
O induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H
induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H O
O . These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
. These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki; Sasaki, Takayuki*; Uehara, Akihiro*; Takeda, Shino*; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Kobayashi, Taishi*
The Chemistry of Thorium, Plutonium and MA, 254 Pages, 2022/03
The chemistry of nuclear materials such as Thorium (Part 1) and Plutonium (Part 2) was described in relation from the fundamentals on solid chemistry and solution chemistry to the practicals on the experiment and evaluation method in detail. Minor actinides such as Neptunium, Americium, Curium and Protoactinium, was introduced the basics on the solid and solution chemistry.
Uehara, Akihiro*; Akiyama, Daisuke*; Ikeda, Atsushi; Numako, Chiya*; Terada, Yasuko*; Nitta, Kiyofumi*; Ina, Toshiaki*; Takeda-Homma, Shino*; Kirishima, Akira*; Sato, Nobuaki*
Journal of Nuclear Materials, 559, p.153422_1 - 153422_11, 2022/02
Times Cited Count:2 Percentile:53.91(Materials Science, Multidisciplinary)Nagai, Takayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Okamoto, Yoshihiro
2020-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Oyobi "Hito, Kankyo To Busshitsu O Tsunagu Inobeshion Soshutsu Dainamikku, Araiansu" Kenkyu Seika, Katsudo Hokokusho (CD-ROM), 1 Pages, 2021/11
no abstracts in English
 O
O ; Application of Raman imaging technique to uranium compound
; Application of Raman imaging technique to uranium compoundKusaka, Ryoji; Kumagai, Yuta; Yomogida, Takumi; Takano, Masahide; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 58(6), p.629 - 634, 2021/06
Times Cited Count:7 Percentile:66.68(Nuclear Science & Technology)Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki; Sasaki, Takayuki*; Uehara, Akihiro*; Takeda, Shino*
Uran No Kagaku (II); Hoho To Jissen, 143 Pages, 2021/03
This book describes necessary facts when readers would have an opportunity to treat Uranium for experiments. In the content, the method section shows experimental facilities and equipment including method, and the practical section mentions solution and solid state experiments using Uranium and/or radioisotopes.
Nagai, Takayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Okamoto, Yoshihiro
2019-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Oyobi "Hito, Kankyo To Busshitsu O Tsunagu Inobeshion Soshutsu Dainamikku, Araiansu" Kenkyu Seika, Katsudo Hokokusho (CD-ROM), P. 20191107_1, 2020/11
no abstracts in English
Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki
Uran No Kagaku (I); Kiso To Oyo, 184 Pages, 2020/06
This particular book deals with fundamental items related with chemistry of Uranium and consists of basic section and practical section. In the basic section, inorganic and radiochemistry of Uranium was described. On the other hand, in the practical section, the process chemistry related with reprocessing of nuclear fuels and "debris" involved with Fukushima Daiichi Power Plant accident were described. This book is intended for use by scientists, engineers and students in the nuclear industry in their education and/or professional practice.
Nagai, Takayuki; Kobayashi, Hidekazu; Okamoto, Yoshihiro; Akiyama, Daisuke*; Sato, Nobuaki*; Uehara, Akihiro*; Fujii, Toshiyuki*; Sekimoto, Shun*
KURNS Progress Report 2018, P. 105, 2019/08
To understand this structural change of a borosilicate glass by a neutron irradiation in detail, the irradiation test was carried out in KUR in 2017FY. The glass structure was estimated by using Raman spectrometry in 2018FY. Comparing with the Raman spectra of glass samples before and after irradiation, it could be observed the change of peak height of Si-O bridging structure by the irradiation.
Nagai, Takayuki; Akiyama, Daisuke*; Sato, Nobuaki*; Okamoto, Yoshihiro
"Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Oyobi "Hito, Kankyo To Busshitsu O Tsunagu Inobeshion Soshutsu Danamikku, Araiansu" Kenkyu Seika, Katsudo Hokokusho (Heisei-30-Nendo) (CD-ROM), P. 20181080_1, 2019/06
no abstracts in English
Kimuro, Shingo; Kirishima, Akira*; Kitatsuji, Yoshihiro; Miyakawa, Kazuya; Akiyama, Daisuke*; Sato, Nobuaki*
Journal of Chemical Thermodynamics, 132, p.352 - 362, 2019/05
Times Cited Count:10 Percentile:44.91(Thermodynamics)A combination of potentiometry and calorimetry was used for the determination of the thermodynamic quantities of complexation of generic and groundwater humic acid (HA), which was isolated from deep groundwater at Horonobe, Hokkaido, Japan, with copper (II) ions and uranyl (VI) ions. The apparent complexation constant of Horonobe HA was independent of the pH, whereas that of generic HA was dependent on the pH. This observation indicates that the polyelectrolyte effect of Horonobe HA is negligible because of its small molecular size. In addition, the effect of the heterogeneity of Horonobe HA was not significant. Moreover, the complexation enthalpy of Horonobe HA was consistent with that of homogeneous poly(acrylic acid), which means the complexation of Horonobe HA was not affected by the functional group heterogeneity. Consequently, the characteristic complexation mechanism of Horonobe HA was revealed based on the determined thermodynamic quantities.
Nagai, Takayuki; Okamoto, Yoshihiro; Akiyama, Daisuke*; Sato, Nobuaki*
Photon Factory Activity Report 2018 (Internet), 2 Pages, 2019/00
no abstracts in English
Kimuro, Shingo*; Kirishima, Akira*; Nagao, Seiya*; Saito, Takumi*; Amano, Yuki; Miyakawa, Kazuya; Akiyama, Daisuke*; Sato, Nobuaki*
Journal of Nuclear Science and Technology, 55(5), p.503 - 515, 2018/05
Times Cited Count:7 Percentile:58.07(Nuclear Science & Technology)no abstracts in English
Nagai, Takayuki; Akiyama, Daisuke*; Sato, Nobuaki*
Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten Kenkyu Seika Hokokusho (Heisei-29-Nendo) (CD-ROM), 1 Pages, 2018/04
no abstracts in English