Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Iwai, Yasunori; Sato, Katsumi; Taniuchi, Junichi*; Noguchi, Hiroshi*; Kubo, Hitoshi*; Harada, Nobuo*; Oshima, Yusuke*; Yamanishi, Toshihiko
Journal of Nuclear Science and Technology, 48(8), p.1184 - 1192, 2011/08
Times Cited Count:34 Percentile:91.82(Nuclear Science & Technology)The inorganic-based hydrophobic Pt-catalyst named H1P has been developed especially for efficient oxidation of a tracer level of tritium in the ambient temperature range even in the presence of saturated water vapor. The overall reaction rate constant for H1P catalyst in the ambient temperature range was considerably larger than that for traditionally applied Pt/Al O
O catalyst. Moreover, the decrease in reaction rate for H1P in the presence of saturated water vapor compared with in the absence of water vapor was slight due to its excellence in hydrophobic performance. Oxidation reaction on the catalyst surface is the rate-controlling step in the ambient temperature range and diffusion in a catalyst substratum above 313 K due to its fine porosity. The overall reaction rate constant in the ambient temperature range was dependent on the space velocity and hydrogen concentration in carrier.
catalyst. Moreover, the decrease in reaction rate for H1P in the presence of saturated water vapor compared with in the absence of water vapor was slight due to its excellence in hydrophobic performance. Oxidation reaction on the catalyst surface is the rate-controlling step in the ambient temperature range and diffusion in a catalyst substratum above 313 K due to its fine porosity. The overall reaction rate constant in the ambient temperature range was dependent on the space velocity and hydrogen concentration in carrier.
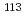 Cd isomer and
Cd isomer and 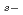 process contribution to rare
process contribution to rare 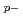 nuclide
nuclide 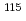 Sn
SnHayakawa, Takehito; Shizuma, Toshiyuki; Chiba, Satoshi; Kajino, Toshitaka*; Hatsukawa, Yuichi; Iwamoto, Nobuyuki; Shinohara, Nobuo; Harada, Hideo
Astrophysical Journal, 707(2), p.859 - 865, 2009/12
Times Cited Count:6 Percentile:20.39(Astronomy & Astrophysics)no abstracts in English
 Cs(n,
Cs(n, )
)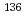 Cs; Fundamental data for the transmutation of nuclear waste
Cs; Fundamental data for the transmutation of nuclear wasteHatsukawa, Yuichi; Shinohara, Nobuo; ; Kobayashi, Katsutoshi; Motoishi, Shoji; Tanase, Masakazu; *; *; Harada, Hideo*
Journal of Radioanalytical and Nuclear Chemistry, 239(3), p.455 - 458, 1999/00
Times Cited Count:2 Percentile:75(Chemistry, Analytical)no abstracts in English
Hayakawa, Takehito; Shizuma, Toshiyuki; Chiba, Satoshi; Kajino, Toshitaka*; Hatsukawa, Yuichi; Iwamoto, Nobuyuki; Shinohara, Nobuo; Harada, Hideo
no journal, ,
no abstracts in English
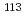 Cd isomer for the study of s-process origin of
Cd isomer for the study of s-process origin of 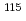 Sn
SnHayakawa, Takehito; Shizuma, Toshiyuki; Kajino, Toshitaka*; Chiba, Satoshi; Hatsukawa, Yuichi; Harada, Hideo; Shinohara, Nobuo
no journal, ,
In order to study the astrophysical origin of a rare isotope 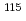 Sn, a neutron capture cross-section for the
Sn, a neutron capture cross-section for the 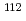 Cd(n,
Cd(n, )
)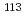 Cd
Cd reaction has been measured with neutrons provided from a nuclear reactor. The cross-section ratio of the isomer to the ground state has been calculated as a function of the incident neutron energy, E, by using a statistical model. The calculated ratios are almost constant over a wide range of E
reaction has been measured with neutrons provided from a nuclear reactor. The cross-section ratio of the isomer to the ground state has been calculated as a function of the incident neutron energy, E, by using a statistical model. The calculated ratios are almost constant over a wide range of E  100 keV. We have evaluated the
100 keV. We have evaluated the 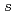 -process contribution to the solar abundance of
-process contribution to the solar abundance of 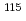 Sn using the classical steady-flow model.
Sn using the classical steady-flow model.
Iwai, Yasunori; Sato, Katsumi; Yamanishi, Toshihiko; Taniuchi, Junichi*; Noguchi, Hiroshi*; Kubo, Hitoshi*; Harada, Nobuo*; Oshima, Yusuke*
no journal, ,
The inorganic-based hydrophobic Pt-catalyst named H1P has been developed especially for efficient oxidation of a tracer level of tritium at room temperature even in the presence of saturated water vapor. Overall reaction rate constant of tritium oxidation on a H1P catalyst in a flow-through system were determined as a function of space velocity, hydrogen concentration in carrier, temperature of catalyst, water vapor concentration in carrier. The overall reaction rate constant for H1P catalyst at room temperature was considerably larger than that for traditionally applied Pt-alumina catalyst. The overall reaction rate constant at room temperature was dependent on the space velocity and hydrogen concentration in carrier. The overall reaction rate constant under the dry and wet conditions was proportional to hydrogen concentration in carrier to the power 0.33 and 0.44, respectively.
Suda, Shoya*; Ishibashi, Kenji*; Lee, E.*; Shigyo, Nobuhiro*; Ikeda, Nobuo*; Sun, G. M.*; Han, B.-Y.*; Takada, Hiroshi; Harada, Masahide
no journal, ,
Our group has experience to carry out the experiment with small electrochemical devices at heavy-water-moderated Advanced Thermal Reactor (ATR, FUGEN), and observed a clear signal increase near the reactor core. The present study is aiming at demonstrating the response of the electrochemical device to tritium amount. The experiments were performed at (1) Pressurized right Water Reactor (PWR, tritium amount of 30 g and a great quantity of beta emitters) at a distance of 26 m from the core, and (2) Tritium Process Laboratory of Japan Atomic Energy Agency (TPL, tritium amount ~10 g) at a position of 8.6 m from the source. Signal increase was observed in both measurements. Analysis made clear that the detector signal is ascribed to the amount of low-energy beta emitters of plutonium 241 and tritium in the PWR experiment while it is attributed to the quantity of tritium in the TPL measurement.