Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 solution in the gigapascal pressure range
solution in the gigapascal pressure rangeYamaguchi, Toshio*; Fukuyama, Nami*; Yoshida, Koji*; Katayama, Yoshinori*; Machida, Shinichi*; Hattori, Takanori
Liquids, 3(3), p.288 - 302, 2023/09
We report the structure of an aqueous 2 mol/kg MgCl solution at pressures from 0.1 MPa to 4 GPa and temperatures from 300 to 500 K revealed by X-ray and neutron scattering measurements. The scattering data are analyzed by empirical potential structure refinement (EPSR) modeling to derive the pair distribution functions, coordination number distributions, angle distributions, and spatial density functions as a function of pressure and temperature. Mg
solution at pressures from 0.1 MPa to 4 GPa and temperatures from 300 to 500 K revealed by X-ray and neutron scattering measurements. The scattering data are analyzed by empirical potential structure refinement (EPSR) modeling to derive the pair distribution functions, coordination number distributions, angle distributions, and spatial density functions as a function of pressure and temperature. Mg forms rigid solvation shells extended to the third shell; the first solvation shell of six-fold octahedral coordination with about six water molecules at 0 GPa transforms into about five water molecules and one Cl
forms rigid solvation shells extended to the third shell; the first solvation shell of six-fold octahedral coordination with about six water molecules at 0 GPa transforms into about five water molecules and one Cl due to the formation of the contact ion pairs in the GPa pressure range. The Cl
due to the formation of the contact ion pairs in the GPa pressure range. The Cl solvation shows a substantial pressure dependence; the coordination number of a water oxygen atom around Cl
solvation shows a substantial pressure dependence; the coordination number of a water oxygen atom around Cl increases from 8 at 0.1 MPa/300 K to 10 at 4 GPa/500 K. The solvent water transforms the tetrahedral network structure at 0.1 MPa/300 K to a densely packed structure in the GPa pressure range; the number of water oxygen atoms around a central water molecule gradually increases from 4.6 at 0.1 MPa/298 K to 8.4 at 4 GPa/500 K.
increases from 8 at 0.1 MPa/300 K to 10 at 4 GPa/500 K. The solvent water transforms the tetrahedral network structure at 0.1 MPa/300 K to a densely packed structure in the GPa pressure range; the number of water oxygen atoms around a central water molecule gradually increases from 4.6 at 0.1 MPa/298 K to 8.4 at 4 GPa/500 K.
Yamaguchi, Toshio*; Yoshida, Koji*; Machida, Shinichi*; Hattori, Takanori
Journal of Molecular Liquids, 365, p.120181_1 - 120181_10, 2022/11
Times Cited Count:1 Percentile:16.81(Chemistry, Physical)Neutron scattering measurements were performed on an aqueous 3 mol/kg NaCl solution in D O at temperature and pressure conditions of 0.1 MPa/298K, 1 GPa/298K, 1 GPa/523K, and 4 GPa/523K. The empirical potential structure refinement method was applied to the obtained data to extract the pair correlation function, coordination number distribution, angular distribution (orientation correlation), and spatial density function (3-D structure). From those results, pressure and temperature dependence of solvation and association of ions and solvent-water structure were discussed.
O at temperature and pressure conditions of 0.1 MPa/298K, 1 GPa/298K, 1 GPa/523K, and 4 GPa/523K. The empirical potential structure refinement method was applied to the obtained data to extract the pair correlation function, coordination number distribution, angular distribution (orientation correlation), and spatial density function (3-D structure). From those results, pressure and temperature dependence of solvation and association of ions and solvent-water structure were discussed.
 /water nanofluid
/water nanofluidYoshida, Koji*; Sanada, Yusuke*; Yamaguchi, Toshio*; Matsuura, Masato*; Tamatsukuri, Hiromu; Uchiyama, Hiroshi*
Journal of Molecular Liquids, 366, p.120218_1 - 120218_9, 2022/11
Times Cited Count:1 Percentile:16.81(Chemistry, Physical)Zhang, W. Q.*; Yamaguchi, Toshio*; Fang, C. H.*; Yoshida, Koji*; Zhou, Y. Q.*; Zhu, F. Y.*; Machida, Shinichi*; Hattori, Takanori; Li, W.*
Journal of Molecular Liquids, 348, p.118080_1 - 118080_11, 2022/02
Times Cited Count:2 Percentile:34.79(Chemistry, Physical)The ion hydration and association and hydrogen-bonded water structure in an aqueous 3 mol/kg RbCl solution were investigated at 298 K/0.1 MPa, 298 K/1 GPa, 523 K/1 GPa, and 523 K/4 GPa by neutron diffraction combined with EPSR methods. The second hydration layer of Rb and Cl
and Cl becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb
becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb (Cl
(Cl ) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb
) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl
and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl does not change very much. The number of contact-ion pairs Rb
does not change very much. The number of contact-ion pairs Rb -Cl
-Cl decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
 -lactoglobulin
-lactoglobulinYoshida, Koji*; Zenin, Tomohiro*; Fujiyoshi, Ayako*; Sanada, Yusuke*; Yamaguchi, Toshio*; Murata, Kunihiko*; Takata, Shinichi; Hiroi, Kosuke; Takahiro, Takekiyo*; Yoshimura, Yukihiro*
Journal of Molecular Liquids, 293, p.111477_1 - 111477_9, 2019/11
Times Cited Count:8 Percentile:39.14(Chemistry, Physical)Yamaguchi, Toshio*; Nishino, Masaaki*; Yoshida, Koji*; Takumi, Masaharu*; Nagata, Kiyofumi*; Hattori, Takanori
European Journal of Inorganic Chemistry, 2019(8), p.1170 - 1177, 2019/02
Times Cited Count:16 Percentile:74.57(Chemistry, Inorganic & Nuclear)Neutron diffraction measurements of an aqueous 2 mol dm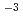 CaCl
CaCl solutions in D
solutions in D O have been made at 1 GPa, 298 K as well as 0.1 MPa, 298 K. The experimental structure factors are subjected to Empirical Potential Structure Refinement (EPSR) modeling to reveal the ion hydration and association and solvent water at the atomic level. About seven water molecules surround Ca
O have been made at 1 GPa, 298 K as well as 0.1 MPa, 298 K. The experimental structure factors are subjected to Empirical Potential Structure Refinement (EPSR) modeling to reveal the ion hydration and association and solvent water at the atomic level. About seven water molecules surround Ca at the Ca-O and Ca-D distances of 2.44
at the Ca-O and Ca-D distances of 2.44  and 3.70
and 3.70  , respectively, at both pressures, suggesting no significant pressure effect on the cation hydration. On the other hand, the Cl
, respectively, at both pressures, suggesting no significant pressure effect on the cation hydration. On the other hand, the Cl ion shows a drastic change in water oxygen coordination from 7 at 0.1 MPa to 14 at 1 GPa, accompanied by shortening of Cl-O distance from 3.18
ion shows a drastic change in water oxygen coordination from 7 at 0.1 MPa to 14 at 1 GPa, accompanied by shortening of Cl-O distance from 3.18  to 3.15
to 3.15  . However, the number of water hydrogen atoms around Cl
. However, the number of water hydrogen atoms around Cl does not change significantly as 6.0
does not change significantly as 6.0  6.7 with shortening Cl-D distance from 2.22 to 2.18
6.7 with shortening Cl-D distance from 2.22 to 2.18  on compression. The pressure effect on the solvent water structure is also drastic as an increase in water oxygen atoms of 4.7 at the O-O distance of 2.79
on compression. The pressure effect on the solvent water structure is also drastic as an increase in water oxygen atoms of 4.7 at the O-O distance of 2.79  at 0.1 MPa to 10.3 at 2.85
at 0.1 MPa to 10.3 at 2.85  at 1 GPa. The number of water hydrogen atoms, however, does not change as 1.2 at the O-D distance of 1.74
at 1 GPa. The number of water hydrogen atoms, however, does not change as 1.2 at the O-D distance of 1.74  for both pressures, demonstrating the presence of the O
for both pressures, demonstrating the presence of the O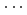 D hydrogen bonds which are significantly bent at 1 GPa at 298 K. This change of hydrogen bonds in water with pressure probably causes the drastic increase in water oxygen atoms around Cl
D hydrogen bonds which are significantly bent at 1 GPa at 298 K. This change of hydrogen bonds in water with pressure probably causes the drastic increase in water oxygen atoms around Cl .
.
Yoshida, Koji*; Inoue, Takuya*; Torigoe, Motokatsu*; Yamada, Takeshi*; Shibata, Kaoru; Yamaguchi, Toshio*
Journal of Chemical Physics, 149(12), p.124502_1 - 124502_10, 2018/09
Times Cited Count:4 Percentile:17.83(Chemistry, Physical)Differential scanning calorimetry, X-ray diffraction, and quasi-elastic neutron scattering (QENS) measurements of aqueous glycine solutions confined in mesoporous silica (MCM-41) were performed at different glycine concentrations, pH, and loading ratio (= mass of glycine solution / mass of dry MCM-41) in the temperature range from 305 to 180 K to discuss the confinement effect on the thermal behavior, the structure, and the dynamic properties of the solutions.
Hosokawa, Shinya*; Kimura, Koji*; Yamasaki, Michiaki*; Kawamura, Yoshihito*; Yoshida, Koji*; Inui, Masanori*; Tsutsui, Satoshi*; Baron, A. Q. R.*; Kawakita, Yukinobu; Ito, Shinichi*
Journal of Alloys and Compounds, 695, p.426 - 432, 2017/02
Times Cited Count:3 Percentile:16.72(Chemistry, Physical)Ito, Shinichi*; Yokoo, Tetsuya*; Masuda, Takatsugu*; Yoshizawa, Hideki*; Soda, Minoru*; Ikeda, Yoichi*; Ibuka, Soshi*; Kawana, Daichi*; Sato, Taku*; Nambu, Yusuke*; et al.
JPS Conference Proceedings (Internet), 8, p.034001_1 - 034001_6, 2015/09
Yamaguchi, Toshio*; Fujimura, Koji*; Uchi, Kazuya*; Yoshida, Koji*; Katayama, Yoshinori
Journal of Molecular Liquids, 176, p.44 - 51, 2012/12
Times Cited Count:18 Percentile:55.59(Chemistry, Physical)X-ray diffraction measurements in an energy-dispersive mode have been made on water under various thermodynamic conditions of 298 K/30 MPa, 473 K/30 MPa and 573 K/30 MPa on a laboratory X-ray diffractometer. All the X-ray structure factors as well as those of water already measured at 298 K/1 GPa, 473 K/0.35 GPa and 486 K/4 GPa by using synchrotron X-ray radiation were subjected to empirical potential structure refinement (EPSR) modeling to reveal the detailed hydrogen bonding features in terms of partial pair correlation function, coordination number and three-dimensional spatial density function as a function of temperature and pressure. It has been shown to what extent the tetrahedral hydrogen-bonded network of water is perturbed by pressure and temperature.
Suzuya, Kentaro; Kameda, Yasuo*; Otomo, Toshiya*; Yoshida, Koji*; Ito, Keiji*; Fukunaga, Toshiharu*; Misawa, Masakatsu*
Journal of Neutron Research, 13(1-3), p.123 - 128, 2005/03
The design, performance, philosophy, lessons learned and advantages of the neutron total scattering spectrometer for hydrogenous materials, FAST, under consideration for the J-PARC-JSNS, are described. In particular, novel instrumentation concept such as the small fractional scattered neutron flight path,  = L
= L /(L
/(L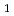 +L
+L ), where L
), where L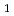 is the incident flight path, moderator to sample, L
is the incident flight path, moderator to sample, L is the scattered neutron flight path from sample to detector, is presented and their expected performance at the JSNS is considered.
is the scattered neutron flight path from sample to detector, is presented and their expected performance at the JSNS is considered.