Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Koyama, Shinichi; ; ; Mitsugashira, Toshiaki; Morozumi, Katsufumi;
Journal of Nuclear Science and Technology, 35(6), 406 Pages, 1998/00
Times Cited Count:13 Percentile:70.95(Nuclear Science & Technology)None
 Np transmutation characteristics with chemical analysis of neptunium dosimeter irradiated in "Joyo"
Np transmutation characteristics with chemical analysis of neptunium dosimeter irradiated in "Joyo"Osaka, Masahiko; Koyama, Shinichi; Otsuka, Yuko; Mitsugashira, Toshiaki; Namekawa, Takashi; Konno, Koichi
PNC TN9410 98-020, 70 Pages, 1997/12
The purpose of this study is to evaluate transmutation characteristics such as dependence of  Np transmutation rate to neutron energy spectrum and neutron fluences. Analysis of the Neptunium dosimeter, in which was irradiated in the experimental fast reactor "Joyo", was carried out by applying the technique for analysis of minor actinide nuclides in irradiated MOX fuel. It is necessary to remove Vanadium before analysis of Neptunium dosimeter because NpO
Np transmutation rate to neutron energy spectrum and neutron fluences. Analysis of the Neptunium dosimeter, in which was irradiated in the experimental fast reactor "Joyo", was carried out by applying the technique for analysis of minor actinide nuclides in irradiated MOX fuel. It is necessary to remove Vanadium before analysis of Neptunium dosimeter because NpO powder was enclosed in Neptunium dosimeter that was made of Vanadium capsule. The result of analysis and evaluations are as follows. (1)In order to recover Neptunium sample completely, sample was dissolved with capsule before removing the Vanadium from sample solution. Sample treatment method of whole capsule dissolution for chemical analysis of Neptunium dosimeter was established. (2)
powder was enclosed in Neptunium dosimeter that was made of Vanadium capsule. The result of analysis and evaluations are as follows. (1)In order to recover Neptunium sample completely, sample was dissolved with capsule before removing the Vanadium from sample solution. Sample treatment method of whole capsule dissolution for chemical analysis of Neptunium dosimeter was established. (2) Np, Plutonium isotopes,
Np, Plutonium isotopes,  Am and
Am and  Cs in the dosimeter were analyzed using the method of whole capsule dissolution, alpha spectrometry, gamma spectrometry and isotopic dilution mass spectrometry. Transmutation rate of
Cs in the dosimeter were analyzed using the method of whole capsule dissolution, alpha spectrometry, gamma spectrometry and isotopic dilution mass spectrometry. Transmutation rate of  Np was calculated using the analyzed value. Tendency of transmutation rate was certified, which is higher fission ratio at the center and higher capture ratio at both upper and lower end. (3)Transmutation rate with error was evaluated by neutron fluences considering the neutron energy spectrum, and calculated value by "MAGI" code was agreed well with analysis value. Dependence of transmutation rate of
Np was calculated using the analyzed value. Tendency of transmutation rate was certified, which is higher fission ratio at the center and higher capture ratio at both upper and lower end. (3)Transmutation rate with error was evaluated by neutron fluences considering the neutron energy spectrum, and calculated value by "MAGI" code was agreed well with analysis value. Dependence of transmutation rate of  Np to neutron energy spectrum was certified.
Np to neutron energy spectrum was certified.
Koyama, Shinichi; ; Osaka, Masahiko; ; ; Mitsugashira, Toshiaki
Proceedings of International Conference on Future Nuclear Systems (Global'97), Vol.2, 0 Pages, 1997/10
no abstracts in English
Osaka, Masahiko; Koyama, Shinichi; Otsuka, Yuko; Mitsugashira, Toshiaki; Konno, Koichi; Kajitani, Yukio
PNC TN9410 96-297, 79 Pages, 1996/11
As a part of evaluation of irradiation behavior and burnup characteristics of MA nuclides such as Np, Am and Cm in MA containing MOX fuel, we are studying the quantitative analysis techniques for MA nuclides in irradiated fuel. In this study, we studied the mutual separation method for Am and Cm to establish the analysis method for Am and Cm following Np analysis by alpha spectrometry. The measurements of Am and Cm are difficult to analyze quantitatively because the amounts of some nuclides are too small and the number of nuclides are large, whose energies of the alpha radioactive rays are almost same. Therefore we selected to analyze the trace amount of Am and Cm isotopes using mass spectrometry, and have studied the techniques for mutual separation of Am and Cm using oxidation method of Am by NaBiO for standard samples. We have also evaluated the availability of this method for irradiated fuel. Results are as follows. (1)Through the mutual separation tests, we have found the most suitable conditions for separation of both Am and Cm from each other element. The method obtaining Am which contains no Cm is used water for precipitation washing solution, containing Cm is achicved that the remaining ratio of Am (ratio of radioactivity of
for standard samples. We have also evaluated the availability of this method for irradiated fuel. Results are as follows. (1)Through the mutual separation tests, we have found the most suitable conditions for separation of both Am and Cm from each other element. The method obtaining Am which contains no Cm is used water for precipitation washing solution, containing Cm is achicved that the remaining ratio of Am (ratio of radioactivity of  Am/
Am/ Cm against before separation) were reduced less than 1/10 for Cm. (2)Applying of this method to irradiated fuel, the coordinate remaining ratio and the chemical yield of Am and Cm were almost same as them in the separation tests. This method to apply various irradiated MOX Fuel is therefore possible. (3)The isotope ratio
Cm against before separation) were reduced less than 1/10 for Cm. (2)Applying of this method to irradiated fuel, the coordinate remaining ratio and the chemical yield of Am and Cm were almost same as them in the separation tests. This method to apply various irradiated MOX Fuel is therefore possible. (3)The isotope ratio  Am,
Am, 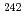 Am and
Am and  Am measured by mass spectrometry, which could not be analyzed by radioactive ray spectrometry causing less than detection limit, were 98.55% : 0.62% : 0.83%. We also measured zero of the mass number of 240 and 244 on the specimens and then certified no contamination of Cm to Am.
Am measured by mass spectrometry, which could not be analyzed by radioactive ray spectrometry causing less than detection limit, were 98.55% : 0.62% : 0.83%. We also measured zero of the mass number of 240 and 244 on the specimens and then certified no contamination of Cm to Am.
 Am content and evaluation of burnup dependence in Am containing MOX fuel pin
Am content and evaluation of burnup dependence in Am containing MOX fuel pinKoyama, Shinichi; Osaka, Masahiko; Otsuka, Yuko; Konno, Koichi; Kajitani, Yukio; Mitsugashira, Toshiaki
PNC TN9410 96-301, 61 Pages, 1996/10
We are studying quantitative analysis of Minor Actinides (Np, Am, Cm) in irradiated fuels as a part of the PNC research project for advanced nuclear fuel recycle system. In alpha-gamma section, irradiation behavior of MOX fuel and burning characteristic evaluation research of the MA nuclide which contain the minor actinide species are carrying out. We measured  Am content of the MOX fuel pin which contained
Am content of the MOX fuel pin which contained  Am of about 0.9wt% before irradiation and were irradiated up to 26.2GWd/t in the JOYO reactor. The results are as follows. Burn-up dependence of the
Am of about 0.9wt% before irradiation and were irradiated up to 26.2GWd/t in the JOYO reactor. The results are as follows. Burn-up dependence of the  Am content in this samples was not observed. The
Am content in this samples was not observed. The  Am content showed the fixed value of about 1% in the range from 0 to 28GWd/t. This reason is assumed that Am produced by
Am content showed the fixed value of about 1% in the range from 0 to 28GWd/t. This reason is assumed that Am produced by  -decay of
-decay of  Pu for cooling times between each cycles valances it in disappearance under irradiated in JOYO based on the calculated value by ORIGEN-II code.
Pu for cooling times between each cycles valances it in disappearance under irradiated in JOYO based on the calculated value by ORIGEN-II code.