Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ohira, Saki; Abe, Takeyasu; Iida, Yoshihisa
Radiochimica Acta, 111(7), p.525 - 531, 2023/07
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)The solubility of 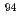 Nb in calcium alkaline solutions is one of the important parameters in safety assessment of intermediate-depth disposal which are assumed to use cementitious materials. Nb solubility and solubility-limiting solid phases of Nb in these systems remain unclear. The oversaturation solubility experiments were performed systematically in the 0.001-0.1 M CaCl
Nb in calcium alkaline solutions is one of the important parameters in safety assessment of intermediate-depth disposal which are assumed to use cementitious materials. Nb solubility and solubility-limiting solid phases of Nb in these systems remain unclear. The oversaturation solubility experiments were performed systematically in the 0.001-0.1 M CaCl solutions under alkali conditions, and the characterization of precipitated solid phase controlling Nb solubility was conducted. The negative dependence of Nb solubilities on pH and Ca concentration was observed in solubility experiments, the molar ratio of Nb to Ca of precipitated solid phase was 0.66. The pH and Ca dependence of Nb solubilities was reproduced by the reaction with Nb aqueous species Nb(OH)
solutions under alkali conditions, and the characterization of precipitated solid phase controlling Nb solubility was conducted. The negative dependence of Nb solubilities on pH and Ca concentration was observed in solubility experiments, the molar ratio of Nb to Ca of precipitated solid phase was 0.66. The pH and Ca dependence of Nb solubilities was reproduced by the reaction with Nb aqueous species Nb(OH)
 and Ca-Nb oxide with the molar ratio of Nb to Ca 0.66, e.g., Ca
and Ca-Nb oxide with the molar ratio of Nb to Ca 0.66, e.g., Ca Nb
Nb O
O (am).
(am).
Ohira, Saki; Iida, Yoshihisa
Proceedings of Waste Management Symposia 2023 (WM2023) (Internet), 10 Pages, 2023/02
The sorption distribution coefficient ( d) of niobium-94 (Nb-94) on minerals is one of the important parameters in safety assessment of radioactive waste disposal. In a previous study, the
d) of niobium-94 (Nb-94) on minerals is one of the important parameters in safety assessment of radioactive waste disposal. In a previous study, the  d values of Nb under alkali condition in the presence of Ca, were two orders of magnitude higher than those in the presence of Na. In this study, Nb sorption experiments were performed to reexamine the effect of Ca on Nb sorption on clay minerals, and blank tests were performed to check for precipitation formation. The results showed that the Nb sorption onto montmorillonite and illite, did not depend on the Ca concentration, and
d values of Nb under alkali condition in the presence of Ca, were two orders of magnitude higher than those in the presence of Na. In this study, Nb sorption experiments were performed to reexamine the effect of Ca on Nb sorption on clay minerals, and blank tests were performed to check for precipitation formation. The results showed that the Nb sorption onto montmorillonite and illite, did not depend on the Ca concentration, and  d values obtained in the presence of Ca were the same as those in the absence of Ca. A sorption model assuming sorption by complexation on the mineral surface was developed and then calculated using the geochemical calculation code. The model with the surface species X_ONb(OH)
d values obtained in the presence of Ca were the same as those in the absence of Ca. A sorption model assuming sorption by complexation on the mineral surface was developed and then calculated using the geochemical calculation code. The model with the surface species X_ONb(OH) and X_ONb(OH)
and X_ONb(OH)
 represented trends in the data obtained.
represented trends in the data obtained.
Shimada, Asako; Taniguchi, Yoshinori; Kakiuchi, Kazuo; Ohira, Saki; Iida, Yoshihisa; Sugiyama, Tomoyuki; Amaya, Masaki; Maruyama, Yu
Scientific Reports (Internet), 12(1), p.2086_1 - 2086_11, 2022/02
Times Cited Count:1 Percentile:31.61(Multidisciplinary Sciences)no abstracts in English
Yamaguchi, Tetsuji; Ohira, Saki; Hemmi, Ko; Barr, L.; Shimada, Asako; Maeda, Toshikatsu; Iida, Yoshihisa
Radiochimica Acta, 108(11), p.873 - 877, 2020/11
Times Cited Count:7 Percentile:66.68(Chemistry, Inorganic & Nuclear)Ohira, Saki
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 27(1), p.34 - 36, 2020/06
no abstracts in English
Yamaguchi, Tetsuji; Hemmi, Ko; Logan, B.*; Shimada, Asako; Ohira, Saki; Iida, Yoshihisa
no journal, ,
We developed a sorption model taking into account the surface species of Ca-Nb-OH, and reanalyzed the data of Ervanne et al. (2014) for illite. As a result, we were able to reproduce the high sorption distribution coefficient of Nb to illite in the presence of Ca.
Shimada, Asako; Taniguchi, Yoshinori; Ohira, Saki; Iida, Yoshihisa
no journal, ,
no abstracts in English
Kakiuchi, Kazuo; Shimada, Asako; Ohira, Saki; Iida, Yoshihisa
no journal, ,
no abstracts in English
Ohira, Saki; Abe, Takeyasu; Iida, Yoshihisa
no journal, ,
Niobium-94 is an important radionuclide in the safety assessment of intermediate depth disposal. Solubility-limiting solid and solution species of Nb under high Ca concentration and alkaline conditions, estimated as cement-equilibrated solutions, remain unclear. The solubility of Nb under high Ca concentration and alkaline conditions was measured, and solid phase was analyzed by scanning electron microscope energy disperse spectrometry and powder X-ray diffraction. The obtained results show that Nb solubility is negatively correlated with Nb and pH or Ca concentration. The results of Nb solubility experiments and solid phase analysis in this study suggest that the Nb solubility would be limited by solubility-limiting solid Ca Nb
Nb O
O (am) and solution species Nb(OH)
(am) and solution species Nb(OH)
 .
.
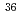 Cl and
Cl and 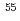 Fe
FeShimada, Asako; Ohira, Saki; Iida, Yoshihisa
no journal, ,
no abstracts in English
Ohira, Saki
no journal, ,
The solubility and sorption distribution coefficient of niobium-94 (Nb-94) are one of the important parameters in safety assessment of radioactive waste disposal. However, the solubility and sorption distribution coefficient of Nb under calcium and alkaline conditions are still unknown. In this study, Nb oversaturation solubility experiments in 0.001-0.1 M calcium chloride solutions were systematically carried out and the Nb solubility-limiting solid phase was evaluated: the Nb concentration showed a negative dependence on pH and Ca concentration and the Ca/Nb molar ratio of the precipitated solid phase was 0.66. The pH and Ca concentration dependence was confirmed to be reproducible in the dissolution reaction between the dissolved species of Nb hydroxide ions and the Ca-Nb solid phase showing a Ca/Nb ratio of 0.66. Sorption tests below solubility showed similar values for the Nb sorption distribution coefficient in the presence and absence of Ca, confirming that Nb sorption can be reproduced by the surface complexation model of Nb hydroxide complexes.