Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 - and K-montmorillonite
- and K-montmorilloniteKawakita, Ryohei; Saito, Akito*; Sakuma, Hiroshi*; Anraku, Sohtaro; Kikuchi, Ryosuke*; Otake, Tsubasa*; Sato, Tsutomu*
Applied Clay Science, 231, p.106722_1 - 106722_7, 2023/01
Times Cited Count:1 Percentile:19.05(Chemistry, Physical)Anraku, Sohtaro; Walker, C.*; Oda, Chie; Mihara, Morihiro; Honda, Akira
Proceedings of 15th International Congress on the Chemistry of Cement (ICCC 2019) (Internet), 11 Pages, 2019/09
Walker, C.*; Anraku, Sohtaro; Oda, Chie; Mihara, Morihiro; Honda, Akira
Proceedings of 15th International Congress on the Chemistry of Cement (ICCC 2019) (Internet), 11 Pages, 2019/09
Anraku, Sohtaro
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 24(1), p.65 - 68, 2017/06
no abstracts in English
Anraku, Sohtaro; Matsubara, Isamu*; Morimoto, Kazuya*; Sato, Tsutomu*
Nendo Kagaku, 55(2), p.17 - 30, 2017/00
Anionic radionuclides are important for the long-term safety assessment of Japanese transuranic (TRU) waste disposal facilities. Degradation of cementitious materials used to construct the TRU waste disposal facilities, however, can produce a hyperalkaline leachate and so it is necessary to understand the reaction mechanisms that will control the behavior and fate of anionic radionuclides under these hyperalkaline conditions. An excellent natural analogue site to study relevant reaction mechanisms is provided in Oman where hyperalkaline spring waters (pH  11) from serpentinized peridotites discharge into moderately alkaline rivers. Aragonite was found in all secondary mineral samples, with accessory minerals of calcite, layered double hydroxide (LDH) and brucite. LDH was observed at the high Al concentration springs and brucite at the low Al concentration springs. Calcite was only found close to the springs. Distal calcite formation was inhibited due to high Mg concentrations in the river water. The spatial distribution of minerals therefore implicates the importance of the mixing ratio of spring to river water and the relative chemical compositions of the spring and river waters. Supporting mixing model calculations could successfully reproduce the precipitation of aragonite and LDH. The observed decrease in Ca concentration could be explained by aragonite precipitation. pH exerted a strong control on the precipitation of LDH and so too, therefore, on Al concentration. In the mixing water experiments containing up to 40% river water, LDH and brucite were both oversaturated, but brucite was not always identified by XRD. The possible inhibition of brucite by LDH precipitation was an unexpected result.
11) from serpentinized peridotites discharge into moderately alkaline rivers. Aragonite was found in all secondary mineral samples, with accessory minerals of calcite, layered double hydroxide (LDH) and brucite. LDH was observed at the high Al concentration springs and brucite at the low Al concentration springs. Calcite was only found close to the springs. Distal calcite formation was inhibited due to high Mg concentrations in the river water. The spatial distribution of minerals therefore implicates the importance of the mixing ratio of spring to river water and the relative chemical compositions of the spring and river waters. Supporting mixing model calculations could successfully reproduce the precipitation of aragonite and LDH. The observed decrease in Ca concentration could be explained by aragonite precipitation. pH exerted a strong control on the precipitation of LDH and so too, therefore, on Al concentration. In the mixing water experiments containing up to 40% river water, LDH and brucite were both oversaturated, but brucite was not always identified by XRD. The possible inhibition of brucite by LDH precipitation was an unexpected result.
 -montmorillonite
-montmorilloniteKawakita, Ryohei*; Saito, Akito*; Sakuma, Hiroshi*; Anraku, Sohtaro; Oda, Chie; Mihara, Morihiro; Sato, Tsutomu*
no journal, ,
no abstracts in English
Kawakita, Ryohei*; Saito, Akito*; Sakuma, Hiroshi*; Anraku, Sohtaro; Oda, Chie; Mihara, Morihiro; Sato, Tsutomu*
no journal, ,
no abstracts in English
Walker, C.; Anraku, Sohtaro; Mitsui, Seiichiro; Oda, Chie; Mihara, Morihiro; Honda, Akira
no journal, ,
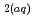 , HSiO
, HSiO
 and SiO
and SiO

Walker, C.*; Anraku, Sohtaro; Oda, Chie; Mitsui, Seiichiro; Mihara, Morihiro
no journal, ,
Equilibrium constants ( ) describing the formation reactions of SiO
) describing the formation reactions of SiO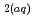 , HSiO
, HSiO
 and SiO
and SiO
 can be used to derive their thermodynamic properties. However, SiO
can be used to derive their thermodynamic properties. However, SiO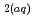 was derived from inaccurate quartz solubility data, HSiO
was derived from inaccurate quartz solubility data, HSiO
 was not extrapolated to zero ionic strength and SiO
was not extrapolated to zero ionic strength and SiO
 is routinely ignored because of its restricted dominance to very high pH
is routinely ignored because of its restricted dominance to very high pH  13 solutions. Using quartz and water data as well known "anchor" points,
13 solutions. Using quartz and water data as well known "anchor" points,  values describing the formation reactions of SiO
values describing the formation reactions of SiO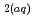 , HSiO
, HSiO
 and SiO
and SiO
 were revised to derive new thermodynamic properties and revised Helgeson-Kirkham-Flowers equation of state (r-H-K-F EoS) parameters. These properties and parameters can be used in the derivation of the thermodynamic properties of other Si bearing aqueous species/complexes and the thermodynamic properties of cementitious, clay, zeolite, and rock forming minerals, and in calculating groundwater compositions relevant to the geological disposal of radioactive wastes.
were revised to derive new thermodynamic properties and revised Helgeson-Kirkham-Flowers equation of state (r-H-K-F EoS) parameters. These properties and parameters can be used in the derivation of the thermodynamic properties of other Si bearing aqueous species/complexes and the thermodynamic properties of cementitious, clay, zeolite, and rock forming minerals, and in calculating groundwater compositions relevant to the geological disposal of radioactive wastes.
Anraku, Sohtaro; Sato, Hisao*; Walker, C.*; Amano, Yuki; Sakurai, Akitaka; Nakayama, Masashi; Tachi, Yukio
no journal, ,
Walker, C.*; Anraku, Sohtaro; Sasamoto, Hiroshi; Mihara, Morihiro
no journal, ,
A thermodynamically credible calcium(-aluminate)-silicate-hydrate (C(-A)-S-H) gel solubility model has to consider a variety of features, including structure, composition, phase boundaries, measured/estimated thermodynamic properties, molar volumes, and, of course, solubility behavior in terms of pH values and Ca, Al and Si concentrations expressed as a function of C(-A)-S-H gel composition, temperature and ionic strength. This study provides an account of the rapidly growing body of data that are concerned with these features and of the more promising approaches that can be used to develop a C(-A)-S-H gel solubility model. JAEA research on the degradation of high content fly ash silica fume cement (HFSC) provides a good working example that highlights the need to develop a C(-A)-S-H gel solubility model.