Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
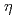
 -semiquinone complex; A Possible dielectric response mechanism
-semiquinone complex; A Possible dielectric response mechanismMitsumi, Minoru*; Ezaki, Kazunari*; Komatsu, Yuki*; Toriumi, Koshiro*; Miyato, Tatsuya*; Mizuno, Motohiro*; Azuma, Nobuaki*; Miyazaki, Yuji*; Nakano, Motohiro*; Kitagawa, Yasutaka*; et al.
Chemistry; A European Journal, 21(27), p.9682 - 9696, 2015/06
Times Cited Count:7 Percentile:25.07(Chemistry, Multidisciplinary)Sasada, Yuto*; Miyazaki, Yuji*; Nakano, Motohiro*; Matsuo, Yusuke*; Walker, C.*; Sasamoto, Hiroshi; Mihara, Morihiro
no journal, ,
Cementitous materials are to be used in large quantities in the geological disposal of highly radioactive TRU waste. Thermodynamic data on cement hydrate minerals are important to model the long-term dissolution behavior of cement materials during the reaction of these materials with groundwater. Portlandite (Ca(OH) ) is a major component (20 to 25 wt%) of hydrated Portland cement. Low temperature heat capacity measurements of three types of portlandite (low purity, high purity, and large crystal) showed purity dependence. The large crystal sample followed Debye's T
) is a major component (20 to 25 wt%) of hydrated Portland cement. Low temperature heat capacity measurements of three types of portlandite (low purity, high purity, and large crystal) showed purity dependence. The large crystal sample followed Debye's T rule at low temperatures, while the high and low purity samples showed upturn of heat capacity, possibly due to difference in absorbed water vapor and/or calcite (CaCO
rule at low temperatures, while the high and low purity samples showed upturn of heat capacity, possibly due to difference in absorbed water vapor and/or calcite (CaCO ) contamination. Heat capacities of the cement hydrates (ettringite:Ca
) contamination. Heat capacities of the cement hydrates (ettringite:Ca (Al(OH)
(Al(OH) )
) (SO
(SO )
) (H
(H O)
O) and monosulfate:Ca
and monosulfate:Ca Al
Al (OH)
(OH) (SO
(SO )(H
)(H O)
O) ) exhibited phase transitions associated with the hydration water.
) exhibited phase transitions associated with the hydration water.