Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Onuki, Toshihiko; Sakamoto, Fuminori; Kozai, Naofumi; Namba, Kenji*; Neda, Hitoshi*; Sasaki, Yoshito; Niizato, Tadafumi; Watanabe, Naoko*; Kozaki, Tamotsu*
Environmental Science; Processes & Impacts, 21(7), p.1164 - 1173, 2019/07
Times Cited Count:10 Percentile:44.69(Chemistry, Analytical)The fate of radioactive Cs deposited after the Fukushima nuclear power plant accident and its associated radiological impacts are largely dependent on its mobility from surface soils to forest ecosystems. We measured the accumulation of radioactive Cs in the fruit bodies of wild fungi in the forest at Iidate, Fukushima, Japan. The transfer factors (TFs) of radioactive Cs from soil to the fruit bodies of wild fungi were between 10  to 10
to 10 , a range similar to those reported for the fruit bodies collected in Europe after the Chernobyl accident and in parts of Japan contaminated by nuclear bomb test fallout. Comparison of the TFs of the wild mushroom and that of the fungal hyphae of 704 stock strains grown on agar medium containing nutrients and radioactive Cs showed that the TFs of wild mushroom were lower. TF was less than 0.1 after addition of the minerals zeolite, vermiculite, phlogopite, smectite, or illite of 1% weight to the agar medium. These results indicate that the presence of minerals decrease Cs uptake by fungi grown in the agar medium.
, a range similar to those reported for the fruit bodies collected in Europe after the Chernobyl accident and in parts of Japan contaminated by nuclear bomb test fallout. Comparison of the TFs of the wild mushroom and that of the fungal hyphae of 704 stock strains grown on agar medium containing nutrients and radioactive Cs showed that the TFs of wild mushroom were lower. TF was less than 0.1 after addition of the minerals zeolite, vermiculite, phlogopite, smectite, or illite of 1% weight to the agar medium. These results indicate that the presence of minerals decrease Cs uptake by fungi grown in the agar medium.
 Cs and Mn accumulator plant,
Cs and Mn accumulator plant, 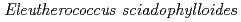 , increase
, increase  Cs and Mn desorption in the soil
Cs and Mn desorption in the soilYamaji, Keiko*; Nagata, Satoshi*; Haruma, Toshikatsu*; Onuki, Toshihiko; Kozaki, Tamotsu*; Watanabe, Naoko*; Namba, Kenji*
Journal of Environmental Radioactivity, 153, p.112 - 119, 2016/03
Times Cited Count:20 Percentile:54.68(Environmental Sciences)Of the 463 strains that we isolated, 107 (23.1%) produced the siderophores. We found  Cs and Mn desorption concomitant with Al and Fe desorption. These results suggest that root endophytes of
Cs and Mn desorption concomitant with Al and Fe desorption. These results suggest that root endophytes of  Cs accumulator plant produce siderophores, resulting in the desorption of
Cs accumulator plant produce siderophores, resulting in the desorption of  Cs from the contaminated soil collected at Fukushima, Japan.
Cs from the contaminated soil collected at Fukushima, Japan.
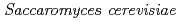
Onuki, Toshihiko; Sakamoto, Fuminori; Yamasaki, Shinya*; Kozai, Naofumi; Shiotsu, Hiroyuki; Utsunomiya, Satoshi*; Watanabe, Naoko*; Kozaki, Tamotsu*
Journal of Environmental Radioactivity, 144, p.127 - 133, 2015/06
Times Cited Count:9 Percentile:27.12(Environmental Sciences)The accumulation of Cs by unicellular fungus of 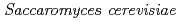 in the presence of minerals has been studied to elucidate the role of microorganisms in the migration of radioactive Cs in the environment. In the presence of minerals in the agar medium, the radioactivity in the yeast cells was in the order of mica
in the presence of minerals has been studied to elucidate the role of microorganisms in the migration of radioactive Cs in the environment. In the presence of minerals in the agar medium, the radioactivity in the yeast cells was in the order of mica  smectite, illite
smectite, illite  vermiculite, phlogopite, zeolite. This order is inversely correlated to the ratio of the concentration of radioactive Cs between the minerals and the medium solution. These results strongly suggest that the yeast accumulates radioactive Cs competitively with minerals.
vermiculite, phlogopite, zeolite. This order is inversely correlated to the ratio of the concentration of radioactive Cs between the minerals and the medium solution. These results strongly suggest that the yeast accumulates radioactive Cs competitively with minerals.
Tanaka, Shingo*; Yokota, Hideharu; Ono, Hirokazu; Nakayama, Masashi; Fujita, Tomoo; Takiya, Hiroaki*; Watanabe, Naoko*; Kozaki, Tamotsu*
Proceedings of 23rd International Conference on Nuclear Engineering (ICONE-23) (DVD-ROM), 6 Pages, 2015/05
Hirano, Fumio; Sato, Seichi*; Kozaki, Tamotsu*; Inagaki, Yaohiro*; Iwasaki, Tomohiko*; Oe, Toshiaki*; Kato, Kazuyuki*; Kitayama, Kazumi*; Nagasaki, Shinya*; Niibori, Yuichi*
Journal of Nuclear Science and Technology, 49(3), p.310 - 319, 2012/03
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)The thermal impacts of hull and end piece wastes from the reprocessing of MOX spent fuels burned in LWRs on repository performance were investigated. The heat generation rates in MOX spent fuels and the resulting heat generation rates in hull and end piece wastes change depending on the fuel histories including the burn-up of UO spent fuels, the cooling period before reprocessing, the storage period of fresh MOX fuels. The heat generation rates of hull and end piece wastes from the reprocessing of MOX spent fuels with any of those histories are significantly larger than those from UO
spent fuels, the cooling period before reprocessing, the storage period of fresh MOX fuels. The heat generation rates of hull and end piece wastes from the reprocessing of MOX spent fuels with any of those histories are significantly larger than those from UO spent fuels with burn-ups of 45 GWd/THM. If a temperature below 80
spent fuels with burn-ups of 45 GWd/THM. If a temperature below 80 C is specified for cement-based materials used in waste packages after disposal, the allowable number of canisters containing compacted hull and end pieces in a package for 45 GWd-MOX needs to be limited to a value of 0.7 to 1.6, which is significantly lower than the value of 4.0 for 45 GWd-UO
C is specified for cement-based materials used in waste packages after disposal, the allowable number of canisters containing compacted hull and end pieces in a package for 45 GWd-MOX needs to be limited to a value of 0.7 to 1.6, which is significantly lower than the value of 4.0 for 45 GWd-UO .
.
 ions in Na- and Ca-montmorillonite mixtures
ions in Na- and Ca-montmorillonite mixturesKozaki, Tamotsu*; Sawaguchi, Takuma; Fujishima, Atsushi; Sato, Seichi*
Physics and Chemistry of the Earth, 35(6-8), p.254 - 258, 2010/00
Times Cited Count:21 Percentile:54.44(Geosciences, Multidisciplinary)Compacted Na-bentonite, of which the major mineral is montmorillonite, is a candidate buffer material for the geological disposal of high-level radioactive waste. A potential alteration of the bentonite in a repository is the partial replacement of the exchangeable cations of Na with Ca
with Ca . The Ca
. The Ca cations could be released from cementitious materials and diffuse into the buffer material in the repository. In this study, to evaluate the alteration that could reduce the performance of the bentonite buffer, the apparent diffusion coefficients of HTO and Ca
cations could be released from cementitious materials and diffuse into the buffer material in the repository. In this study, to evaluate the alteration that could reduce the performance of the bentonite buffer, the apparent diffusion coefficients of HTO and Ca ions were determined from non-steady, one-dimensional diffusion experiments using Na- and Ca-montmorillonite mixtures with different ionic equivalent fractions of Ca
ions were determined from non-steady, one-dimensional diffusion experiments using Na- and Ca-montmorillonite mixtures with different ionic equivalent fractions of Ca ions. The apparent diffusion coefficient of HTO at a dry density of 1.0 Mg m
ions. The apparent diffusion coefficient of HTO at a dry density of 1.0 Mg m slightly increased with an increase in the ionic equivalent fraction of Ca
slightly increased with an increase in the ionic equivalent fraction of Ca ions. However, the apparent diffusion coefficient of Ca
ions. However, the apparent diffusion coefficient of Ca and the activation energy for diffusion at the same dry density were independent of the ionic equivalent fraction of Ca
and the activation energy for diffusion at the same dry density were independent of the ionic equivalent fraction of Ca ions. These findings suggest that unlike HTO, which can be postulated to diffuse mainly in pore water, Ca
ions. These findings suggest that unlike HTO, which can be postulated to diffuse mainly in pore water, Ca ion diffusion could occur predominantly in interlayer spaces.
ion diffusion could occur predominantly in interlayer spaces.

 O and applying electric potential gradient
O and applying electric potential gradientTanaka, Shingo*; Noda, Natsuko*; Higashihara, Tomohiro*; Sato, Seichi*; Kozaki, Tamotsu*; Sato, Haruo; Hatanaka, Koichiro
Physics and Chemistry of the Earth, 33(Suppl.1), p.S163 - S168, 2008/00
In order to identify mass transport pathway in compacted bentonite, water transport behavior in compacted montmorillonite which is the major clay mineral constituent of the bentonite was studied. Back-to-back diffusion and electro-osmosis experiments for H O were carried out at montmorillonite densities of 1.0, 1.2 and 1.4 Mg/m
O were carried out at montmorillonite densities of 1.0, 1.2 and 1.4 Mg/m using H
using H
 O as a tracer. Apparent diffusivities from the diffusion experiments and advection velosities and hydraulic dispersities from the electro-osmosis experiments were determined. The mass transport pathways were discussed by comparing with concentration profiles and peak positions of He, Na and Cl which were reported in the past. The hydraulic dispersities decreased in the order of He, H
O as a tracer. Apparent diffusivities from the diffusion experiments and advection velosities and hydraulic dispersities from the electro-osmosis experiments were determined. The mass transport pathways were discussed by comparing with concentration profiles and peak positions of He, Na and Cl which were reported in the past. The hydraulic dispersities decreased in the order of He, H O, Cl and Na, and these differences were considered to be due to that transport pathway depended on species and hydraulic dispersity for each species also depended on transport pathway.
O, Cl and Na, and these differences were considered to be due to that transport pathway depended on species and hydraulic dispersity for each species also depended on transport pathway.
 -type montmorillonite; Potential chemical species of iron contaminant
-type montmorillonite; Potential chemical species of iron contaminantKozai, Naofumi; Inada, Koichi*; Adachi, Yoshifusa*; Kawamura, Sachi*; Kashimoto, Yusuke*; Kozaki, Tamotsu*; Sato, Seichi*; Onuki, Toshihiko; Sakai, Takuro; Sato, Takahiro; et al.
Journal of Solid State Chemistry, 180(8), p.2279 - 2289, 2007/08
Times Cited Count:14 Percentile:48.19(Chemistry, Inorganic & Nuclear)Fe -montmorillonite with Fe
-montmorillonite with Fe ions occupying cation exchange sites is an ideal transformation product in bentonite buffer material. We previously prepared a Fe
ions occupying cation exchange sites is an ideal transformation product in bentonite buffer material. We previously prepared a Fe -montmorillonite sample using a FeCl
-montmorillonite sample using a FeCl solution under an inert gas condition. This study attempted to determine the potential contaminant iron chemical species in the sample. It was found that a small amount of Cl
solution under an inert gas condition. This study attempted to determine the potential contaminant iron chemical species in the sample. It was found that a small amount of Cl ions remained dispersed throughout the sample. The Cl
ions remained dispersed throughout the sample. The Cl ion retention may be due to the adsorption of FeCl
ion retention may be due to the adsorption of FeCl in the initial FeCl
in the initial FeCl solution and the subsequent containment of the Cl
solution and the subsequent containment of the Cl ions that are dissociated from the FeCl
ions that are dissociated from the FeCl during excess salt removal treatment. The latter may be explained by the slow release of the remaining Cl
during excess salt removal treatment. The latter may be explained by the slow release of the remaining Cl ions from the collapsed interlayer of the montmorillonite or the transformation of a minor fraction of the remaining FeCl
ions from the collapsed interlayer of the montmorillonite or the transformation of a minor fraction of the remaining FeCl to iron (III) hydroxide chloride complexes having low solubility.
to iron (III) hydroxide chloride complexes having low solubility.
Takano, Masahide; Tagami, Susumu; Minato, Kazuo; Kozaki, Tamotsu*; Sato, Seichi*
Journal of Alloys and Compounds, 439(1-2), p.215 - 220, 2007/07
Times Cited Count:16 Percentile:64.54(Chemistry, Physical)ZrN is a possible candidate for the diluent material of the nitride fuel containing minor actinides. In the present study, the lattice thermal expansions of ZrN, DyN and (Dy,Zr)N solid solutions were measured by high temperature XRD, as the analogous fuel material. The average linear thermal expansion coefficients of these nitrides increased from 7.86 10
10 to 9.54
to 9.54 10
10 K
K with increasing Dy fraction. On the analogy with the results on the composition dependence of the thermal expansion coefficients, the preferable effect of ZrN as the diluent material is suggested.
with increasing Dy fraction. On the analogy with the results on the composition dependence of the thermal expansion coefficients, the preferable effect of ZrN as the diluent material is suggested.
Manjanna, J.*; Kozaki, Tamotsu*; Kozai, Naofumi; Sato, Seichi*
Journal of Nuclear Science and Technology, 44(7), p.929 - 932, 2007/07
Times Cited Count:18 Percentile:75.3(Nuclear Science & Technology)This study developed a new preparation method of Fe(II)-montmorillonite. In this method, first, a Fe(II)-NTA complex solution was prepared by dissolving iron oxides with NTA and a reducing agent. Next, montmorillonite was immersed in this Fe(II)-NTA solution to allow montmorillonite adsorb Fe ions. The advantages of this method are (1) inert-gas atmosphere is not needed for the entire preparation process and (2) no anions such as Cl
ions. The advantages of this method are (1) inert-gas atmosphere is not needed for the entire preparation process and (2) no anions such as Cl ions, which form ion pairs with Fe
ions, which form ion pairs with Fe ions, are not included in all the chemicals used.
ions, are not included in all the chemicals used.
Kozaki, Tamotsu*; Sato, Seichi*
JNC TJ8400 2004-022, 39 Pages, 2005/02
Compacted bentonite is considered as a candidate buffer material in the geological disposal of high-level radioactive waste. An ssential function of the compacted bentonite is to retard the transport of radionuclides from waste forms to the surrounding host rock after degradation of an overpack. Therefore, diffusion behavior of radionuclides in the compacted bentonite is one of the important issues to be studied for the safety assessment of the geological disposal. In this study, through-diffusion experiments were performed for sodium ions in compacted Na-montmorillonite, since sodium ions are the major exchangeable cations of the Na-montmorillonite which may affect the diffusion behaviors of radionuclides. Cumulate fluxes of Na ions diffusing into the montmorillonite (inlet) and out of the montmorillonite (outlet) were monitored as a function of time at the diffusion experiments under different diffusion temperature. The best-fitted parameters of effective diffusion coefficient and capacity factor to the cumulate fluxes of outlet were obtained by the analysis with or without the consideration of isotopic dilution of
Na ions diffusing into the montmorillonite (inlet) and out of the montmorillonite (outlet) were monitored as a function of time at the diffusion experiments under different diffusion temperature. The best-fitted parameters of effective diffusion coefficient and capacity factor to the cumulate fluxes of outlet were obtained by the analysis with or without the consideration of isotopic dilution of Na in the diffusion system. However, the parameters could not fit to the cumulate flux of inlet. This suggests that there must be still unknown diffusion process in the diffusion system.
Na in the diffusion system. However, the parameters could not fit to the cumulate flux of inlet. This suggests that there must be still unknown diffusion process in the diffusion system.
Kozaki, Tamotsu*; Sato, Seichi*
JNC TJ8400 2003-075, 34 Pages, 2004/03
Compacted bentonite is considered as a candidate buffer material in the geological disposal of high-level radioactive waste. An important function of the compacted bentonite is to retard the transport of radionuclides from waste forms to the surrounding host rock after degradation of an overpack. Therefore, diffusion behavior of radionuclides in the compacted bentonite has been extensively studied by many researchers for the performance assessments of the geological disposal. However, diffusion mechanism of radionuclides in the bentonite cannot be fully understood, and most experimental data have been obtained at room temperature for the bentonite saturated with low salinity water, which would disagree often with real repository conditions. In this study, therefore, apparent diffusion coefficients were determined at various diffusion temperatures for chloride ions in Na-montmorillonite samples saturated with NaCl solution of high salinity. Activation energies for the apparent diffusion were also obtained from the temperature dependents of the diffusion coefficients at different salinity. As the salinity increased, the apparent diffusion coefficients of chloride ions in montmorillonite were found to increase slightly. On the other hand, the activation energies for the chloride diffusion were found to be almost constant (approximately 12 kJ mol ) and less than that in free water (17.4 kJ mol
) and less than that in free water (17.4 kJ mol ). Effects of salinity on diffusion behavior of radionuclides in montmorillonite werediscussed from the viewpoints of microstructure of montmorillonite and distribution of ions in the montmorillonite. As a result, the diffusion behavior of sodium ions could be explained by the changes of the predominant diffusion process among pore water diffusion, surface diffusion, and interlayer diffusion that could be caused by the increase of salinity.
). Effects of salinity on diffusion behavior of radionuclides in montmorillonite werediscussed from the viewpoints of microstructure of montmorillonite and distribution of ions in the montmorillonite. As a result, the diffusion behavior of sodium ions could be explained by the changes of the predominant diffusion process among pore water diffusion, surface diffusion, and interlayer diffusion that could be caused by the increase of salinity.
*
JNC TJ8400 2002-053, 93 Pages, 2003/02
Diffusion behavior of radionuclides in compacted bentonite plays an important role in the performance assessment of bentonite buffer material in geological disposal of high-level radioactive waste. Microstructure of bentonite is considered to be one of the key parameters to affect on the diffusion behavior. In this study, the montmorillonite samples with two different grain sizes were prepared, and were characterized by several methods, such as the BET and the EGME methods for the specific surface area measurement, the SEM and the AFM observations of sample surfaces, the laser diffraction/scattering particle size analysis for determination of grain size distribution, and the Microfocus X-ray computerized tomography (Micro-CT) for three-dimensional observation of the internal microstructure. The images obtained by Micro-CT were analyzed with original computer software to evaluate grain size, grain shape, and tortuosity factor of diffusion path for radionuclides. The apparent and effective diffusion coefficients of HTO, Cl , and Cs
, and Cs were determined using the montmorillonite samples with different particle sizes and dry densities. The grain size effects on the both diffusion coefficients were found for these radionuclides. On the other hand, the apparent diffusion coefficients of Na
were determined using the montmorillonite samples with different particle sizes and dry densities. The grain size effects on the both diffusion coefficients were found for these radionuclides. On the other hand, the apparent diffusion coefficients of Na and Sr
and Sr ions were determined in the mixture of Na-montmorillonite and silica sands. The effect of the silica sand on the activation energy was found for the both radionuclides in the mixture samples at a specific dry density. In addition, the apparent diffusion coefficients of Na
ions were determined in the mixture of Na-montmorillonite and silica sands. The effect of the silica sand on the activation energy was found for the both radionuclides in the mixture samples at a specific dry density. In addition, the apparent diffusion coefficients of Na ions were determined for compacted Na-montmorillonite saturated with NaCl solution. The apparent diffusion coefficient of Na
ions were determined for compacted Na-montmorillonite saturated with NaCl solution. The apparent diffusion coefficient of Na ions slightly increased as the NaCl concentration increased from 0 to 0.5 M. However, the activation energy for diffusion was found to be 14, 23, and 17 kJ mol
ions slightly increased as the NaCl concentration increased from 0 to 0.5 M. However, the activation energy for diffusion was found to be 14, 23, and 17 kJ mol at NaCl concentrations of 0, 0.1, and 0.5 M, respectively. These unique changes ...
at NaCl concentrations of 0, 0.1, and 0.5 M, respectively. These unique changes ...
Ohashi, Hiroshi*; Sato, Seichi*; Kozaki, Tamotsu*
JAERI-Tech 2002-021, 52 Pages, 2002/03
no abstracts in English
*
JNC TJ8400 2001-044, 32 Pages, 2002/02
Diffusion behavior of radionuclides in compacted bentonite plays an important role in the performance assessment of bentonite buffer material in geological disposal of high-level radioactive waste. Microstructure of bentonite is considered to be one of the key parameters to affect on the diffusion behavior. In this study, therefore, nondestructive, three-dimensional images of the internal microstructures of compacted bentonite samples in dry and water-saturated states were examined with the Microfocus X-ray computerized tomography (Micro-CT). It was confirmed that, for dry bentonite samples, clear high-resolution, three-dimensional images could be obtained by Micro-CT. In addition, such microstructures can easily be quantitatively evaluated if the image data are analyzed with computer graphics. For water-saturated bentonite samples, structural changes in bentonite grains were found. This suggests that outer part of bentonite grains swelled and formed a gel that occupied the vacancies between grains, whereas the inner part of the grains did not change significantly in the water-saturated process. For the mixtures of montmorillonite and silica sand, the effect of silica sand on the diffusion behavior and microstructure of montmorillonite was studied. The diffusion coefficients of HTO and sodium ions in the mixtures of montmorillonite and silica sand were determined.The apparent diffusion coefficients of sodium ions and HTO in the mixture samples were almost the same as those in the pure montmorillonite samples at the same partial dry densities. This suggests that the effect of the silica sand on the diffusion coefficients could be negligible. However, activation energy dependence on the partial dry density for the diffusion of sodium ions in the mixture samples was found to be different from that in the pure montmorillonite samples. This implies that the diffusion process of sodium ions can be altered by the addition of silica sand. Based on the basal spacing ...
Kozai, Naofumi; Adachi, Yoshifusa*; Kawamura, Sachi*; Inada, Koichi*; Kozaki, Tamotsu*; Sato, Seichi*; Ohashi, Hiroshi*; Onuki, Toshihiko; Bamba, Tsunetaka
Journal of Nuclear Science and Technology, 38(12), p.1141 - 1143, 2001/12
This study briefly reports characteristics of Fe-montmorillonite. Fe-montmorillonite was used as a simulant of buffer material in which corrosion products of carbon steel overpack, Fe , were diffused. We have found that this clay retains Se(VI) for which natural montmorillonite, such as Na+-type and Ca
, were diffused. We have found that this clay retains Se(VI) for which natural montmorillonite, such as Na+-type and Ca -type, has little retentivity.
-type, has little retentivity.
Takebe, Shinichi; Kimura, Hideo; Matsuzuru, Hideo; Takahashi, Tomoyuki*; Yasuda, Hiroshi*; Uchida, Shigeo*; Mahara, Yasunori*; Saeki, Akiyoshi*; Sasaki, Noriyuki*; Ashikawa, Nobuo*; et al.
JAERI-Review 2001-015, 81 Pages, 2001/05
no abstracts in English
*; *; *
JNC TJ8400 2001-005, 42 Pages, 2001/03
Diffusion behavior of radionuclides in compacted bentonite plays an important role in the performance assessment of bentonite buffer material in geological disposal of high-level radioactive waste. Microstructure of bentonite is considered to be one of the key parameters to affect on the diffusion behavior. In this study, therefore, two kinds of montmorillonite (major clay mineral of bentonite) with different particle sizes were prepared, and characterized with several methods. In addition, the apparent and effective diffusion coefficients of HTO, Cl , and Cs
, and Cs were determined using the montmorillonite samples with different particle sizes and dry densities. In the sample characterization, the specific surface areas of montmorillonite samples with different particle sizes were determined by the BET and the EGME methods, and the particle size distributions of each sample were analyzed by laser diffraction/scatterring particle size analysis. Microstructure of the samples was also observed by scanning electron microscopy (SEM) and atomic force microscopy (AFM). The BET method gave a higher specific surface area of the fine grained sample than of the coarse sample, while the EGME method gave same values for both samples. The laser diffraction/scattering particle size analysis using ethanol as a dispersion medium gave different particle size distributions, but when the samples were dispersed in water with Na
were determined using the montmorillonite samples with different particle sizes and dry densities. In the sample characterization, the specific surface areas of montmorillonite samples with different particle sizes were determined by the BET and the EGME methods, and the particle size distributions of each sample were analyzed by laser diffraction/scatterring particle size analysis. Microstructure of the samples was also observed by scanning electron microscopy (SEM) and atomic force microscopy (AFM). The BET method gave a higher specific surface area of the fine grained sample than of the coarse sample, while the EGME method gave same values for both samples. The laser diffraction/scattering particle size analysis using ethanol as a dispersion medium gave different particle size distributions, but when the samples were dispersed in water with Na (PO
(PO )
) , the particle size distributions were similar. These findings indicate that the montmorillonite layers, which compose the montmorillonite particles, have the same size, even if the particle sizes of the samples are different. In the diffusion experiments, it was found that the apparent diffusion coefficients of HTO and Cl
, the particle size distributions were similar. These findings indicate that the montmorillonite layers, which compose the montmorillonite particles, have the same size, even if the particle sizes of the samples are different. In the diffusion experiments, it was found that the apparent diffusion coefficients of HTO and Cl for the fine grained sample were higher than for the coarse grained sample at two dry densities, 1.0 and 1.8 Mg m
for the fine grained sample were higher than for the coarse grained sample at two dry densities, 1.0 and 1.8 Mg m , while the opposite particle size effect was observed for Cs
, while the opposite particle size effect was observed for Cs ions. These ...
ions. These ...
Kozai, Naofumi; Inada, Koichi*; Kozaki, Tamotsu*; Sato, Seichi*; Ohashi, Hiroshi*; Bamba, Tsunetaka
Journal of Contaminant Hydrology, 47(2-4), p.149 - 158, 2001/02
Times Cited Count:15 Percentile:41.97(Environmental Sciences)no abstracts in English
*; *; *
JNC TJ8400 2000-018, 79 Pages, 2000/02
As a basic research for geological disposal of high-level radioactive wastes, diffusion behavior of radionuclides and corrosion behavior of overpack materials in clay buffer materials (bentonite) were studied. In the study on the diffusion behavior of radionuclides, basal spacing and water content were determined for water saturated, compacted Na-montmorillonite that is major clay mineral of bentonite. The apparent diffusion coefficients of Na , Sr
, Sr , Cs
, Cs and Cl
and Cl ions and their activation energies were also determined at different dry densities of montmorillonite. For all kinds of ions, the activation energies were found to increase as the dry density increased. These findings suggest that the diffusion mechanism of ions in compacted montmorillonite changed with increasing dry density. As a reasonable explanation for the changes in the activation energy, we proposed a multiprocess diffusion model, in which predominant diffusion process is considered to change from pore water diffusion to interlayer diffusion via surface diffusion when the dry density increases. The Na-montmorillonite is expected to alter by the ion exchange with Ca
ions and their activation energies were also determined at different dry densities of montmorillonite. For all kinds of ions, the activation energies were found to increase as the dry density increased. These findings suggest that the diffusion mechanism of ions in compacted montmorillonite changed with increasing dry density. As a reasonable explanation for the changes in the activation energy, we proposed a multiprocess diffusion model, in which predominant diffusion process is considered to change from pore water diffusion to interlayer diffusion via surface diffusion when the dry density increases. The Na-montmorillonite is expected to alter by the ion exchange with Ca ions, which could be introduced from groundwater and/or cementitious materials in a repository. The apparent diffusion coefficients of Na
ions, which could be introduced from groundwater and/or cementitious materials in a repository. The apparent diffusion coefficients of Na and Cs
and Cs ions and their activation energies were studied for Na/Ca montmorillonite mixtures in order to know the effect of this kind of alteration on the diffusion behavior of ions. It was found that the alteration of montmorillonite affected diffusion coefficients and the activation energies for both kinds of cations. These effects cannot be explained only by the pore water diffusion. The multiprocess diffusion model proposed in this study is suggested as the most reasonable explanation for the effects. The oxidation behavior of pyrite in bentonite during drying process was studied for understanding corrosion behavior of overpack materials in bentonite. There ...
ions and their activation energies were studied for Na/Ca montmorillonite mixtures in order to know the effect of this kind of alteration on the diffusion behavior of ions. It was found that the alteration of montmorillonite affected diffusion coefficients and the activation energies for both kinds of cations. These effects cannot be explained only by the pore water diffusion. The multiprocess diffusion model proposed in this study is suggested as the most reasonable explanation for the effects. The oxidation behavior of pyrite in bentonite during drying process was studied for understanding corrosion behavior of overpack materials in bentonite. There ...