Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamaguchi, Akiko; Kurihara, Yuichi*; Nagata, Kojiro*; Tanaka, Kazuya; Higaki, Shogo*; Kobayashi, Toru; Tanida, Hajime; Ohara, Yoshiyuki*; Yokoyama, Keiichi; Yaita, Tsuyoshi; et al.
Journal of Colloid and Interface Science, 661, p.317 - 332, 2024/05
Times Cited Count:0no abstracts in English
 -Ga
-Ga O
O (001)
(001)Tsai, Y. H.*; Kobata, Masaaki; Fukuda, Tatsuo; Tanida, Hajime; Kobayashi, Toru; Yamashita, Yoshiyuki*
Applied Physics Letters, 124(11), p.112105_1 - 112105_5, 2024/03
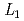 -edge XAFS measurement
-edge XAFS measurementTsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 11(4), p.214 - 217, 2023/08
no abstracts in English
Ouchi, Kazuki; Matsumura, Daiju; Tsuji, Takuya; Kobayashi, Toru; Otobe, Haruyoshi; Kitatsuji, Yoshihiro
RSC Advances (Internet), 13(24), p.16321 - 16326, 2023/05
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)We clarified the chemical state transformation of deposits following the reduction of uranyl ion (U O
O
 ) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U
) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U is formed by the disproportionation of U
is formed by the disproportionation of U . (2) U
. (2) U forms U
forms U hydroxide deposits, and (3) finally, the hydroxide deposits transform into U
hydroxide deposits, and (3) finally, the hydroxide deposits transform into U oxide, generally having a larger electrical resistance than the former.
oxide, generally having a larger electrical resistance than the former.
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:24.43(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Tsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru; Yaita, Tsuyoshi
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 11(1), p.15 - 18, 2023/02
no abstracts in English
Sasaki, Yuji; Nakase, Masahiko*; Kaneko, Masashi; Kobayashi, Toru; Takeshita, Kenji*; Matsumiya, Masahiko*
Analytical Sciences, 5 Pages, 2023/00
Times Cited Count:0 Percentile:0(Chemistry, Analytical)We conducted three field researches on Ru-extraction, XANES, and DFT-calculation. The order of the distribution ratio, D(Ru), from acid, HCl  H
H SO
SO
 HNO
HNO
 HClO
HClO , by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide
, by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide  MIDOA
MIDOA  IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
 Se
Se from multilayer VSe
from multilayer VSe films via V- and Se-desorption
films via V- and Se-desorptionSumida, Kazuki; Kusaka, Shotaro*; Takeda, Yukiharu; Kobayashi, Katsuyoshi*; Hirahara, Toru*
Physical Review B, 106(19), p.195421_1 - 195421_7, 2022/11
Times Cited Count:0 Percentile:0(Materials Science, Multidisciplinary) molecular dynamics simulations reveal the hydration structure of the radium(II) ion
molecular dynamics simulations reveal the hydration structure of the radium(II) ionYamaguchi, Akiko; Nagata, Kojiro*; Kobayashi, Keita; Tanaka, Kazuya; Kobayashi, Toru; Tanida, Hajime; Shimojo, Kojiro; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
iScience (Internet), 25(8), p.104763_1 - 104763_12, 2022/08
Times Cited Count:9 Percentile:68.46(Multidisciplinary Sciences)no abstracts in English
Matsuda, Shohei; Yokoyama, Keiichi; Yaita, Tsuyoshi; Kobayashi, Toru; Kaneta, Yui; Simonnet, M.; Sekiguchi, Tetsuhiro; Honda, Mitsunori; Shimojo, Kojiro; Doi, Reisuke; et al.
Science Advances (Internet), 8(20), p.eabn1991_1 - eabn1991_11, 2022/05
Times Cited Count:6 Percentile:58.16(Multidisciplinary Sciences)no abstracts in English
Yamaguchi, Akiko; Nagata, Kojiro*; Tanaka, Kazuya; Kobayashi, Keita; Kobayashi, Toru; Shimojo, Kojiro; Tanida, Hajime; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
Hosha Kagaku, (45), p.28 - 30, 2022/03
no abstracts in English
Kimata, Tetsuya*; Kakitani, Kenta*; Yamamoto, Shunya*; Shimoyama, Iwao; Matsumura, Daiju; Iwase, Akihiro*; Mao, W.*; Kobayashi, Tomohiro*; Yamaki, Tetsuya*; Terai, Takayuki*
Physical Review Materials (Internet), 6(3), p.035801_1 - 035801_7, 2022/03
Times Cited Count:7 Percentile:71.37(Materials Science, Multidisciplinary) -VSe
-VSe film revealed by element-specific X-ray magnetic circular dichroism
film revealed by element-specific X-ray magnetic circular dichroismSumida, Kazuki; Takeda, Yukiharu; Kusaka, Shotaro*; Kobayashi, Katsuyoshi*; Hirahara, Toru*
Physical Review Materials (Internet), 6(1), p.014006_1 - 014006_8, 2022/01
Times Cited Count:6 Percentile:66.14(Materials Science, Multidisciplinary)We investigated the intrinsic magnetic properties of the two-dimensional ferromagnetic candidate monolayer 1 -VSe
-VSe films using X-ray magnetic circular dichroism (XMCD), an element-specific magnetic probe. By performing high-resolution measurements, we succeeded in detecting a clear XMCD signal from the atomically thin 1
films using X-ray magnetic circular dichroism (XMCD), an element-specific magnetic probe. By performing high-resolution measurements, we succeeded in detecting a clear XMCD signal from the atomically thin 1 -VSe
-VSe films under an external magnetic field. Through manipulation of the X-ray incidence angle, we were able to disentangle the in-plane and out-of-plane magnetic properties and found a strong magnetic anisotropy. Moreover, magnetic field- and temperature-dependent XMCD revealed that there is no long-range ferromagnetic ordering even at 6 K, but short-range ferromagnetic and antiferromagnetic interactions between neighboring vanadium ions exist. Such low-temperature magnetic behavior signifies that the monolayer 1
films under an external magnetic field. Through manipulation of the X-ray incidence angle, we were able to disentangle the in-plane and out-of-plane magnetic properties and found a strong magnetic anisotropy. Moreover, magnetic field- and temperature-dependent XMCD revealed that there is no long-range ferromagnetic ordering even at 6 K, but short-range ferromagnetic and antiferromagnetic interactions between neighboring vanadium ions exist. Such low-temperature magnetic behavior signifies that the monolayer 1 -VSe
-VSe is on the verge of ferromagnetism, and this fact accounts for the reported ferromagnetism in VSe
is on the verge of ferromagnetism, and this fact accounts for the reported ferromagnetism in VSe -based heterostructures.
-based heterostructures.
 through water-soluble Ti complexes as raw material for controlling particle size and distribution of synthesized BaTiO
through water-soluble Ti complexes as raw material for controlling particle size and distribution of synthesized BaTiO nanocubes
nanocubesNakashima, Koichi*; Hironaka, Kota*; Ouchi, Kazuma*; Ajioka, Mao*; Kobayashi, Yoshio*; Yoneda, Yasuhiro; Yin, S.*; Kakihana, Masato*; Sekino, Toru*
ACS Omega (Internet), 6(48), p.32517 - 32527, 2021/12
Times Cited Count:5 Percentile:38.19(Chemistry, Multidisciplinary)The BaTiO  nanoparticles synthesized by the hydrothermal method is examined by synchrotron X-ray and TEM observation. It was found that the particle size of synthesized BaTiO
nanoparticles synthesized by the hydrothermal method is examined by synchrotron X-ray and TEM observation. It was found that the particle size of synthesized BaTiO depends on the particle size of the raw material of TiO
depends on the particle size of the raw material of TiO . Succeeded in synthesizing 100 nm size BaTiO
. Succeeded in synthesizing 100 nm size BaTiO nanocrystals showing uniform particle size distribution using TiO
nanocrystals showing uniform particle size distribution using TiO nanoparticles with optimized particle size. The obtained BaTiO
nanoparticles with optimized particle size. The obtained BaTiO nanocrystal is a tetragonal system of ferroelectric phase. A three-dimensional structure of BaTiO
nanocrystal is a tetragonal system of ferroelectric phase. A three-dimensional structure of BaTiO nanoparticles could be obtained by the electron beam tomography.
nanoparticles could be obtained by the electron beam tomography.
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:2 Percentile:21.56(Chemistry, Inorganic & Nuclear)no abstracts in English
Simonnet, M.; Kobayashi, Toru; Shimojo, Kojiro; Yokoyama, Keiichi; Yaita, Tsuyoshi
Inorganic Chemistry, 60(17), p.13409 - 13418, 2021/09
Times Cited Count:15 Percentile:87.32(Chemistry, Inorganic & Nuclear) nanocubes via precise solvothermal crystal growth and their anomalous surface compositional reconstruction
nanocubes via precise solvothermal crystal growth and their anomalous surface compositional reconstructionNakashima, Koichi*; Onagi, Kaito*; Kobayashi, Yoshio*; Ishigaki, Toru*; Ishikawa, Yoshihisa*; Yoneda, Yasuhiro; Yin, S.*; Kakihana, Masato*; Sekino, Toru*
ACS Omega (Internet), 6(14), p.9410 - 9425, 2021/04
Times Cited Count:9 Percentile:59.91(Chemistry, Multidisciplinary)We succeeded in synthesizing barium titanate nanocubes by hydrothermal synthesis. When the nanocubes were examined in detail using a scanning electron microscope, pulsed neutrons, and large synchrotron radiation, it was found that a reconstituted layer of titanium oxide was formed on the surface.
Hirahara, Toru*; Otrokov, M. M.*; Sasaki, Taisuke*; Sumida, Kazuki*; Tomohiro, Yuta*; Kusaka, Shotaro*; Okuyama, Yuma*; Ichinokura, Satoru*; Kobayashi, Masaki*; Takeda, Yukiharu; et al.
Nature Communications (Internet), 11, p.4821_1 - 4821_8, 2020/09
Times Cited Count:41 Percentile:93.11(Multidisciplinary Sciences)Sugiura, Yuki; Tomura, Tsutomu*; Ishidera, Takamitsu; Doi, Reisuke; Francisco, P. C. M.; Shiwaku, Hideaki; Kobayashi, Toru; Matsumura, Daiju; Takahashi, Yoshio*; Tachi, Yukio
Journal of Radioanalytical and Nuclear Chemistry, 324(2), p.615 - 622, 2020/05
Times Cited Count:4 Percentile:45.45(Chemistry, Analytical)Simonnet, M.; Suzuki, Shinichi; Miyazaki, Yuji*; Kobayashi, Toru; Yokoyama, Keiichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 38(4), p.430 - 440, 2020/00
Times Cited Count:18 Percentile:69.88(Chemistry, Multidisciplinary)