Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H
O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H SO
SO (0.15-0.69 M)/HNO
(0.15-0.69 M)/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of  Rf on cation-exchange resin decreases with increasing [HSO
Rf on cation-exchange resin decreases with increasing [HSO
 ], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H
O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H SO
SO /HNO
/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
 ], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
 SO
SO with Aliquat336 as homologues of seaborgium (Sg)
with Aliquat336 as homologues of seaborgium (Sg)Mitsukai, Akina; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Kaneya, Yusuke; Takeda, Shinsaku*; Nagame, Yuichiro; Komori, Yukiko*; Murakami, Masashi*; et al.
no journal, ,
We have started studying sulphate-complex formation of a transactinide element, seaborgium (Sg). In this study, we report on the extraction behavior of carrier-free radioisotopes 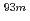 Mo and
Mo and  W which are lighter homologs of Sg, from aqueous H
W which are lighter homologs of Sg, from aqueous H SO
SO solution with amine extractant, Aliquat336, dissolved in toluene by a batch method. These radioisotopes were produced in the
solution with amine extractant, Aliquat336, dissolved in toluene by a batch method. These radioisotopes were produced in the 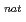 Zr(
Zr( ,
,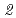 )
)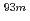 Mo and
Mo and  Ta(
Ta(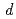 ,x
,x )
) W reactions at the RIKEN K70 AVF cyclotron. Results of the extraction experiments showed that the distribution ratios of Mo and W increase sharply above ~3.0 M H
W reactions at the RIKEN K70 AVF cyclotron. Results of the extraction experiments showed that the distribution ratios of Mo and W increase sharply above ~3.0 M H SO
SO . Based on the slope analysis, it was indicated that anionic sulphate-complex of [MO
. Based on the slope analysis, it was indicated that anionic sulphate-complex of [MO (SO
(SO )
) ]
] (M = Mo, W) are formed in
(M = Mo, W) are formed in  5 M H
5 M H SO
SO . These results suggest that the present system is applicable to the extraction of Sg.
. These results suggest that the present system is applicable to the extraction of Sg.