Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Niwa, Hideharu*; Saito, Makoto*; Kobayashi, Masaki*; Harada, Yoshihisa*; Oshima, Masaharu*; Moriya, Shogo*; Matsubayashi, Katsuyuki*; Nabae, Yuta*; Kuroki, Shigeki*; Ikeda, Takashi; et al.
Journal of Power Sources, 223, p.30 - 35, 2013/02
Times Cited Count:18 Percentile:50.94(Chemistry, Physical)To design non-platinum, inexpensive, but high performance carbon-based cathode catalysts for polymer electrolyte fuel cells, it is important to elucidate the active site for oxygen reduction reaction (ORR). However, it is difficult to directly identify the active site by applying conventional structural or electronic probes to such complex systems. Here, we used C 1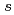 X-ray absorption spectroscopy (XAS) to observe electronic structure of carbon in iron phthalocyanine-based catalysts, and found a signature of edge exposure below the
X-ray absorption spectroscopy (XAS) to observe electronic structure of carbon in iron phthalocyanine-based catalysts, and found a signature of edge exposure below the 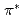 edge, whose intensity is well correlated with the ORR activity. These results demonstrate that C 1
edge, whose intensity is well correlated with the ORR activity. These results demonstrate that C 1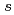 XAS can be used to characterize the ORR activity of carbon-based cathode catalysts in terms of the edge exposure.
XAS can be used to characterize the ORR activity of carbon-based cathode catalysts in terms of the edge exposure.
Kobayashi, Masaki*; Niwa, Hideharu*; Saito, Makoto*; Harada, Yoshihisa*; Oshima, Masaharu*; Ofuchi, Hironori*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; et al.
Electrochimica Acta, 74, p.254 - 259, 2012/07
Times Cited Count:52 Percentile:80.98(Electrochemistry)The electronic structure of the residual metal atoms in FePc-based carbon catalysts, prepared by pyrolyzing a mixture of FePc and phenolic resin polymer at 800 C, before and after acid washing have been investigated using XAFS spectroscopy to clarify the role of Fe in the ORR activity. The decomposition analyses for the XAFS spectra reveal that the composition ratio of each Fe component is unaltered by the acid washing, indicating that the residual Fe components were removed by the acid washing irrespective of their chemical states. Because the oxygen reduction potential was approximately unchanged by the acid washing, the residual Fe itself does not seem to contribute directly to the ORR activity. The residual Fe can act as a catalyst to accelerate the growth of the sp
C, before and after acid washing have been investigated using XAFS spectroscopy to clarify the role of Fe in the ORR activity. The decomposition analyses for the XAFS spectra reveal that the composition ratio of each Fe component is unaltered by the acid washing, indicating that the residual Fe components were removed by the acid washing irrespective of their chemical states. Because the oxygen reduction potential was approximately unchanged by the acid washing, the residual Fe itself does not seem to contribute directly to the ORR activity. The residual Fe can act as a catalyst to accelerate the growth of the sp carbon network during pyrolysis. The results imply that light elements are components of the ORR active sites in the FePc-based carbon catalysts.
carbon network during pyrolysis. The results imply that light elements are components of the ORR active sites in the FePc-based carbon catalysts.
Kobayashi, Masaki*; Niwa, Hideharu*; Harada, Yoshihisa*; Horiba, Koji*; Oshima, Masaharu*; Ofuchi, Hironori*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; et al.
Journal of Power Sources, 196(20), p.8346 - 8351, 2011/10
Times Cited Count:32 Percentile:67.33(Chemistry, Physical)The electronic structure of Co atoms in CoPc-based carbon catalysts, which were prepared by pyrolyzing a mixture of CoPc and phenol resin polymer up to 1000 C, has been investigated using XAFS analysis and HXPES. The Co K XAFS spectra show that most of the Co atoms are in the metallic state and small quantities of oxidized Co components are present in the samples even after acid washing to remove Co atoms. Based on the difference in probing depth between XAFS and HXPES, it was found that after acid washing, the surface region with the aggregated Co clusters is primarily composed of metallic Co. Since the electrochemical properties remain almost unchanged even after the acid washing process, the residual metallic and oxidized Co atoms themselves will hardly contribute to the ORR activity of the CoPc-based carbon cathode catalysts, implying that the active sites of the CoPc-based catalysts primarily consist of light elements such as C and N.
C, has been investigated using XAFS analysis and HXPES. The Co K XAFS spectra show that most of the Co atoms are in the metallic state and small quantities of oxidized Co components are present in the samples even after acid washing to remove Co atoms. Based on the difference in probing depth between XAFS and HXPES, it was found that after acid washing, the surface region with the aggregated Co clusters is primarily composed of metallic Co. Since the electrochemical properties remain almost unchanged even after the acid washing process, the residual metallic and oxidized Co atoms themselves will hardly contribute to the ORR activity of the CoPc-based carbon cathode catalysts, implying that the active sites of the CoPc-based catalysts primarily consist of light elements such as C and N.
Niwa, Hideharu*; Kobayashi, Masaki*; Horiba, Koji*; Harada, Yoshihisa*; Oshima, Masaharu*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; Miyata, Seizo*; et al.
Journal of Power Sources, 196(3), p.1006 - 1011, 2011/02
Times Cited Count:90 Percentile:91.52(Chemistry, Physical)We report on the electronic structure of three different types of N-containing carbon-based cathode catalysts for polymer electrolyte fuel cells observed by hard X-ray photoemission spectroscopy. C 1s spectra show the importance of 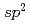 carbon network formation for the oxygen reduction reaction (ORR) activity. Samples having high oxygen reduction reaction activity in terms of oxygen reduction potential contain high concentration of graphite-like nitrogen. Based on a quantitative analysis of our results, the oxygen reduction reaction activity of the carbon-based cathode catalysts will be improved by increasing concentration of graphite-like nitrogen in a developed
carbon network formation for the oxygen reduction reaction (ORR) activity. Samples having high oxygen reduction reaction activity in terms of oxygen reduction potential contain high concentration of graphite-like nitrogen. Based on a quantitative analysis of our results, the oxygen reduction reaction activity of the carbon-based cathode catalysts will be improved by increasing concentration of graphite-like nitrogen in a developed 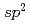 carbon network.
carbon network.
Ikeda, Takashi; Boero, M.*; Huang, S.-F.*; Terakura, Kiyoyuki*; Oshima, Masaharu*; Ozaki, Junichi*; Miyata, Seizo*
Journal of Physical Chemistry C, 114(19), p.8933 - 8937, 2010/05
Times Cited Count:62 Percentile:83.04(Chemistry, Physical)Carbon Alloy Catalysts (CACs) have been attracting a growing interest as potential Pt-free electrode catalysts for polymer electrolyte fuel cell. In this computational study, we inspect possible oxygen adsorption and reduction processes on various models for exposed edges of these catalysts via first principles molecular dynamics. Our simulations suggest that the codoping of boron and nitrogen in CACs is a promising route to further enhancement of their catalytic activity with respect to both stability and reactivity.
Huang, S.-F.*; Terakura, Kiyoyuki*; Ozaki, Taisuke*; Ikeda, Takashi; Boero, M.*; Oshima, Masaharu*; Ozaki, Junichi*; Miyata, Seizo*
Physical Review B, 80(23), p.235410_1 - 235410_12, 2009/12
Times Cited Count:168 Percentile:97.31(Materials Science, Multidisciplinary)Recent studies suggest that the carbon-alloy catalyst with doped nitrogen may be a powerful candidate for cathode catalyst of fuel cell. In this paper, we aim to clarify the microscopic mechanisms of the enhancement in the catalyst activity caused by nitrogen doping using a simple graphene cluster model. We analyze modifications in the electronic structures and the energetical stability for some different configurations of N doping. We extend the analysis to the case of co-doping of nitrogen and boron and propose two possible scenarios explaining the further enhancement of catalytic activity by N and B co-doping.
Niwa, Hideharu*; Horiba, Koji*; Harada, Yoshihisa*; Oshima, Masaharu*; Ikeda, Takashi; Terakura, Kiyoyuki*; Ozaki, Junichi*; Miyata, Seizo*
Journal of Power Sources, 187(1), p.93 - 97, 2009/02
Times Cited Count:434 Percentile:99.8(Chemistry, Physical)The electronic structure of nitrogens introduced in various carbon-based cathode catalysts for a polymer electrolyte fuel cell (PEFC) has been investigated using X-ray absorption spectroscopy (XAS). The profile of the 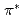 peaks at the pre-edge of the N 1s XAS spectra is used to determine the chemical states of nitrogens, which can be a marker of the oxygen reduction reaction (ORR) activity; it is found that catalysts that have relatively high amount of graphite-like nitrogen exhibit higher ORR activity than those having relatively high amount of pyridine-like nitrogen. We propose that effective doping of graphite-like nitrogen is a practical guideline for the synthesis of active carbon alloy catalysts.
peaks at the pre-edge of the N 1s XAS spectra is used to determine the chemical states of nitrogens, which can be a marker of the oxygen reduction reaction (ORR) activity; it is found that catalysts that have relatively high amount of graphite-like nitrogen exhibit higher ORR activity than those having relatively high amount of pyridine-like nitrogen. We propose that effective doping of graphite-like nitrogen is a practical guideline for the synthesis of active carbon alloy catalysts.
Ikeda, Takashi; Boero, M.*; Huang, S.-F.*; Terakura, Kiyoyuki*; Oshima, Masaharu*; Ozaki, Junichi*
Journal of Physical Chemistry C, 112(38), p.14706 - 14709, 2008/09
Times Cited Count:450 Percentile:99.39(Chemistry, Physical)Nitrogen-doped carbon-based catalysts are attracting a renovated interest as potential Pt-free electrode catalysts for polymer electrolyte fuel cell. In this computational study, we inspect possible oxygen adsorption and reduction processes on various models for the exposed edges of these catalysts. The dynamics of an O molecule solvated in water, mimicking the cathode environment, shows that O
molecule solvated in water, mimicking the cathode environment, shows that O adsorption depends on the morphology and atomic structure of the system. We show that carbon alloys with N dopants at specific sites can exhibit a metal-free catalytic activity.
adsorption depends on the morphology and atomic structure of the system. We show that carbon alloys with N dopants at specific sites can exhibit a metal-free catalytic activity.
Ikeda, Takashi; Boero, M.*; Terakura, Kiyoyuki*; Oshima, Masaharu*; Ozaki, Junichi*
no journal, ,
no abstracts in English
Ikeda, Takashi; Boero, M.*; Huang, S.-F.*; Terakura, Kiyoyuki*; Oshima, Masaharu*; Ozaki, Junichi*; Miyata, Seizo*
no journal, ,
no abstracts in English
Kannari, Naokatsu*; Ozaki, Junichi*; Yamamoto, Shunya; Hakoda, Teruyuki; Yamaki, Tetsuya
no journal, ,
no abstracts in English