Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanaka, Kazuya; Yamaji, Keiko*; Masuya, Hayato*; Tomita, Jumpei; Ozawa, Mayumi*; Yamasaki, Shinya*; Tokunaga, Kohei; Fukuyama, Kenjin*; Ohara, Yoshiyuki*; Maamoun, I.*; et al.
Chemosphere, 355, p.141837_1 - 141837_11, 2024/05
In this study, biogenic Mn(IV) oxide was applied to remove Ra from mine water collected from a U mill tailings pond in the Ningyo-toge center. Just 7.6 mg of biogenic Mn(IV) oxide removed more than 98% of the  Ra from 3 L of mine water, corresponding to a distribution coefficient of 10
Ra from 3 L of mine water, corresponding to a distribution coefficient of 10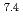 mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
Fueda, Kazuki*; Komiya, Tatsuki*; Minomo, Kenta*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Shiotsu, Hiroyuki; Onuki, Toshihiko*; Grambow, B.*; Law, G. T. W.*; et al.
Chemosphere, 328, p.138566_1 - 138566_12, 2023/07
Times Cited Count:1 Percentile:52.26(Environmental Sciences) C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticles
C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticlesFueda, Kazuki*; Takami, Ryu*; Minomo, Kenta*; Morooka, Kazuya*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Saito, Takumi*; Shiotsu, Hiroyuki; Onuki, Toshihiko*; et al.
Journal of Hazardous Materials, 428, p.128214_1 - 128214_10, 2022/04
Times Cited Count:6 Percentile:68.71(Engineering, Environmental)Tanaka, Kazuya; Yamasaki, Shinya*
Chikyu Kagaku, 55(4), p.93 - 95, 2021/12
Ten years have passed since the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident. In this special issue, we compiled review papers on the environmental behavior of the FDNPP-derived radionuclides from various research fields. This special issue shows that various research fields contributed to better understandings on the environmental behavior of the FDNPP-derived radionuclides.

 on Ni-Zn layered hydroxide salt and its application to removal of ReO
on Ni-Zn layered hydroxide salt and its application to removal of ReO
 as a surrogate of TcO
as a surrogate of TcO

Tanaka, Kazuya; Kozai, Naofumi; Yamasaki, Shinya*; Onuki, Toshihiko; Kaplan, D. I.*; Grambow, B.
Applied Clay Science, 182, p.105282_1 - 105282_8, 2019/12
Times Cited Count:16 Percentile:67.61(Chemistry, Physical)In this study, Ni-Zn layered hydroxide salt (LHS) was used for adsorption experiments of ReO
 , as a surrogate of TcO
, as a surrogate of TcO
 , in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86
, in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86  10
10 eq/g) by fitting adsorption isotherm of ReO
eq/g) by fitting adsorption isotherm of ReO
 to Langmuir plot. The adsorption of ReO
to Langmuir plot. The adsorption of ReO
 at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl
at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl , NO
, NO
 and SO
and SO
 in solution. EXAFS analysis indicated that ReO
in solution. EXAFS analysis indicated that ReO
 was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
 than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
 by reductive precipitation.
by reductive precipitation.
 investigation of radioactive Cs mobility around litter zone in contaminated forest using spent mushroom substrata
investigation of radioactive Cs mobility around litter zone in contaminated forest using spent mushroom substrataOnuki, Toshihiko*; Sakamoto, Fuminori; Kozai, Naofumi; Yamasaki, Shinya*; Sasaki, Yoshito; Niizato, Tadafumi
Journal of Nuclear Science and Technology, 56(9-10), p.814 - 821, 2019/09
Times Cited Count:2 Percentile:21.58(Nuclear Science & Technology)We used the spent mushroom substrata (SMSs) which are a kind of by-product after growing edible mushrooms for the  investigation of radioactive Cs mobility in litter zone in a forest of Fukushima prefecture, Japan. The powder SMS was filled in a plastic net bag of 0.35
investigation of radioactive Cs mobility in litter zone in a forest of Fukushima prefecture, Japan. The powder SMS was filled in a plastic net bag of 0.35 0.55 m, then was placed in a forest for
0.55 m, then was placed in a forest for  6 months under three kinds of different conditions without treatment (No treatment), covered with wooden box (With box), and with zeolite placed on upper position of ground surface (With zeolite). We determined the ratio of radioactivity (TF) in the SMS to that of the soil and litter beneath the SMS bags. TFs of "No treatment" and of "With zeolite" were determined between
6 months under three kinds of different conditions without treatment (No treatment), covered with wooden box (With box), and with zeolite placed on upper position of ground surface (With zeolite). We determined the ratio of radioactivity (TF) in the SMS to that of the soil and litter beneath the SMS bags. TFs of "No treatment" and of "With zeolite" were determined between  0.01 and
0.01 and  0.05 for 6 months. On the other hand, TFs of "With box" were lower by one order at 2 and 4 months than those of "No treatment" and of "With zeolite", and nearly the same values as TFs of "No treatment" and "With zeolite" at 6 months. These results clearly indicate that radioactive Cs accumulates in SMS mainly by throughfall. In addition, for a period of several months, fungi contribute to the accumulation of radioactive Cs in the litter zone, even though radioactive Cs was tightly associated with the soil.
0.05 for 6 months. On the other hand, TFs of "With box" were lower by one order at 2 and 4 months than those of "No treatment" and of "With zeolite", and nearly the same values as TFs of "No treatment" and "With zeolite" at 6 months. These results clearly indicate that radioactive Cs accumulates in SMS mainly by throughfall. In addition, for a period of several months, fungi contribute to the accumulation of radioactive Cs in the litter zone, even though radioactive Cs was tightly associated with the soil.
Kuwahara, Akira; Aiba, Yasuaki*; Yamasaki, Shinya*; Nankawa, Takuya; Matsui, Makoto*
Journal of Analytical Atomic Spectrometry, 33(7), p.1150 - 1153, 2018/07
Times Cited Count:7 Percentile:49(Chemistry, Analytical)Although high-temperature plasma sources have been used for direct isotope analysis of solid samples, the spectral resolution of diode laser absorption spectroscopy in high-temperature plasma is limited by the Doppler broadening of atomic absorption lines. Thus, a decrease in translational temperature is necessary to enhance the spectral resolution and distinguish isotope shifts due to mass number. In this study, a supersonic plasma wind tunnel, also called an arc-jet plasma wind tunnel, was used to enhance spectral resolution drastically, and a demonstration was carried out using natural stable xenon isotopes. As a result, the temperature was found to be about 180 K and the spectral resolution was about one order of magnitude higher than that of the conventional high-temperature source. Additionally, the method proposed herein was verified by using two xenon isotopes.
Tanaka, Kazuya; Watanabe, Naoko*; Yamasaki, Shinya*; Sakaguchi, Aya*; Fan, Q.*; Takahashi, Yoshio*
Geochemical Journal, 52(2), p.173 - 185, 2018/00
Times Cited Count:9 Percentile:43.3(Geochemistry & Geophysics)We analyzed riverbed sediments collected at two sites, Yamakiya and Kuroiwa, in Fukushima after the Fukushima accident. The size distributions of K, Rb, and  Csreflected the mineralogy of sediments, where primary host minerals for these alkali elements would be biotite, K-feldspar, and clay minerals. Silt-size fractions contained high
Csreflected the mineralogy of sediments, where primary host minerals for these alkali elements would be biotite, K-feldspar, and clay minerals. Silt-size fractions contained high  Cs and
Cs and  Cs concentrations possibly due to adsorption on clay minerals. Their concentrations decreased with particle size at the Yamakiya site. In contrast, coarse and very coarse sand fractions from the Kuroiwa site showed higher
Cs concentrations possibly due to adsorption on clay minerals. Their concentrations decreased with particle size at the Yamakiya site. In contrast, coarse and very coarse sand fractions from the Kuroiwa site showed higher  Cs and
Cs and  Cs concentrations in comparison to fine - medium sand fractions. The coarse sand fractions contained many weathered biotite grains. Overall, the size distributions of
Cs concentrations in comparison to fine - medium sand fractions. The coarse sand fractions contained many weathered biotite grains. Overall, the size distributions of  Cs and
Cs and  Cs were similar in the sediments, suggesting that the Fukushima-derived radiocesium was distributed into each particle size fraction in response to the distribution of the stable Cs that was controlled by mineralogical composition.
Cs were similar in the sediments, suggesting that the Fukushima-derived radiocesium was distributed into each particle size fraction in response to the distribution of the stable Cs that was controlled by mineralogical composition.
Yamasaki, Shinya*; Tanaka, Kazuya; Kozai, Naofumi; Onuki, Toshihiko
Applied Geochemistry, 78, p.279 - 286, 2017/03
Times Cited Count:4 Percentile:18.68(Geochemistry & Geophysics)This study examined the rate constant for the U(VI) reduction process by three flavin analogues, which are redox-active biomolecules secreted from anaerobic bacteria, to elucidate their substituent group effect on the U(VI) reduction rate by electrochemical methods. The formation of the U(IV) was monitored by UV-vis spectrometry in the presence of the flavins. The rate constant for the U(VI) reduction by the flavins was determined. The apparent reduction potential of U(VI) increased about 0.2 V in the presence of the mediators, which strongly suggests that the biological electron mediator makes the U(VI) reduction possible even under more oxidative conditions.
Yamasaki, Shinya*; Imoto, Jumpei*; Furuki, Genki*; Ochiai, Asumi*; Onuki, Toshihiko; Sueki, Keisuke*; Namba, Kenji*; Ewing, R. C.*; Utsunomiya, Satoshi*
Science of the Total Environment, 551-552, p.155 - 162, 2016/05
Times Cited Count:32 Percentile:70.18(Environmental Sciences)Cesium-137 ( Cs) of estuary sediment impacted by the FDNPP was measured. Increasing radioactivity was observed from surface to bottom. 90% of the
Cs) of estuary sediment impacted by the FDNPP was measured. Increasing radioactivity was observed from surface to bottom. 90% of the  Cs was strongly bound to clay minerals in the estuary sediments. These results suggest that
Cs was strongly bound to clay minerals in the estuary sediments. These results suggest that  Cs is being transported from contaminated paddy fields to the estuary.
Cs is being transported from contaminated paddy fields to the estuary.
Yu, Q.; Onuki, Toshihiko; Tanaka, Kazuya; Kozai, Naofumi; Yamasaki, Shinya*; Sakamoto, Fuminori; Tani, Yukinori*
Geochimica et Cosmochimica Acta, 174, p.1 - 12, 2016/02
Times Cited Count:18 Percentile:58.95(Geochemistry & Geophysics)Although microorganisms possess high sorption capability for lanthanides (Lns), their biological response affecting Lns migration is unclear. We investigated the effects of microbial activity on transformation of Lns by contact of Lns with Aeremonium strictum under metabolically active condition with Mn(II). A biomolecule that specifically complex to Ce(IV) was found to be released from the fungal cell, facilitating the desorption of Ce(IV) from Mn oxide. This biomolecule was not associated with any other trivalent Lns or Fe, which differed from those non-nuclide-specific organic substances released from resting cells, as reported previously.
Kaneko, Makoto*; Iwata, Hajime; Shiotsu, Hiroyuki; Masaki, Shota*; Kawamoto, Yuji*; Yamasaki, Shinya*; Nakamatsu, Yuki*; Imoto, Jumpei*; Furuki, Genki*; Ochiai, Asumi*; et al.
Frontiers in Energy Research (Internet), 3, p.37_1 - 37_10, 2015/09
The mobility of the aggregates of submicron-sized sheet aluminosilicate in the surface environment is a key factor controlling the current Cs migration in Fukushima.
Onuki, Toshihiko; Jiang, M.*; Sakamoto, Fuminori; Kozai, Naofumi; Yamasaki, Shinya*; Yu, Q.; Tanaka, Kazuya; Utsunomiya, Satoshi*; Xia, X.*; Yange, K.*; et al.
Geochimica et Cosmochimica Acta, 163, p.1 - 13, 2015/08
Times Cited Count:20 Percentile:57.4(Geochemistry & Geophysics)The association of Ce(III) with the microbial cell surface and the formation of Ce phosphate nano-particles are responsible for suppressing the oxidation of Ce(III) to Ce(IV) in the mixtures.
Onuki, Toshihiko; Sakamoto, Fuminori; Yamasaki, Shinya*; Kozai, Naofumi; Shiotsu, Hiroyuki; Utsunomiya, Satoshi*; Watanabe, Naoko*; Kozaki, Tamotsu*
Journal of Environmental Radioactivity, 144, p.127 - 133, 2015/06
Times Cited Count:9 Percentile:27.12(Environmental Sciences)The accumulation of Cs by unicellular fungus of 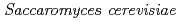 in the presence of minerals has been studied to elucidate the role of microorganisms in the migration of radioactive Cs in the environment. In the presence of minerals in the agar medium, the radioactivity in the yeast cells was in the order of mica
in the presence of minerals has been studied to elucidate the role of microorganisms in the migration of radioactive Cs in the environment. In the presence of minerals in the agar medium, the radioactivity in the yeast cells was in the order of mica  smectite, illite
smectite, illite 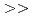 vermiculite, phlogopite, zeolite. This order is inversely correlated to the ratio of the concentration of radioactive Cs between the minerals and the medium solution. These results strongly suggest that the yeast accumulates radioactive Cs competitively with minerals.
vermiculite, phlogopite, zeolite. This order is inversely correlated to the ratio of the concentration of radioactive Cs between the minerals and the medium solution. These results strongly suggest that the yeast accumulates radioactive Cs competitively with minerals.
Kozai, Naofumi; Yamasaki, Shinya; Onuki, Toshihiko
Journal of Radioanalytical and Nuclear Chemistry, 299(3), p.1571 - 1579, 2014/03
Times Cited Count:4 Percentile:30.92(Chemistry, Analytical)To elucidate the sorption behavior of americium(III) on bentonite simplified desorption experiments were applied to the solid phases collected after the sorption experiments. The sorption-desorption behavior was examined in the final pH range from 2 to 8. The desorption experiments revealed that most of the Am was sorbed on the montmorillonite moiety of the bentonite. The sorption of Am on montmorillonite was divided into two types: one was the "exchangeable" sorption, in which the sorbed Am was desorbed with a 1M KCl aqueous solution, and the rest was the "unexchangeable" sorption. The exchangeable sorption was ion exchange of mostly Am . The unexchangeable sorption was the strong sorption of Am hydroxides. An accessory iron mineral, pyrite, might be involved in the Am sorption on bentonite at neutral pH.
. The unexchangeable sorption was the strong sorption of Am hydroxides. An accessory iron mineral, pyrite, might be involved in the Am sorption on bentonite at neutral pH.
Kozai, Naofumi; Yamasaki, Shinya; Onuki, Toshihiko
Journal of Radioanalytical and Nuclear Chemistry, 299(3), p.1581 - 1587, 2014/03
Times Cited Count:2 Percentile:16.44(Chemistry, Analytical)To elucidate migration behaviors of radionuclides in the subsurface environment, this paper investigated sorption-desorption behavior of neptunium(V) on montmorillonite -based two-mineral systems. In montmorillonite-calcite system, the sorption on the montmorillonite moiety decreased with increasing calcite content due to interference by the calcium ions dissolved from the calcite moiety, while Np was not accumulated to the calcite. Total Np sorption on montmorillonite-apatite system was larger than that on apatite-free montmorillonite, but the sorption on the montmorillonite moiety in this system was less than that on apatite-free montmorillonite. Under weakly acid and neutral pH conditions, Np accumulated on the apatite moiety. At final pH  4, though the apatite moiety completely dissolved, the sorption increased with time and the increased Np was was not cation exchangeable. This increase of the unexchangeable sorption cannot be explained by the knowledge accumulated so far.
4, though the apatite moiety completely dissolved, the sorption increased with time and the increased Np was was not cation exchangeable. This increase of the unexchangeable sorption cannot be explained by the knowledge accumulated so far.
Sakamoto, Fuminori; Onuki, Toshihiko; Kozai, Naofumi; Yamasaki, Shinya; Yoshida, Zenko*; Namba, Kenji*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 12(4), p.257 - 266, 2013/12
no abstracts in English
Yamasaki, Shinya; Shirai, Osamu*; Kano, Kenji*; Kozai, Naofumi; Sakamoto, Fuminori; Onuki, Toshihiko
Chemistry Letters, 42(8), p.819 - 821, 2013/08
Times Cited Count:5 Percentile:24.26(Chemistry, Multidisciplinary)The adsorption behavior of lanthanide ions (except for Pm) on liposomes composed of phosphatidylcholine and cholesterol was examined to understand the interaction between lanthanide ions and the phosphoryl moiety of phospholipids. The adsorption of lanthanide ions increased with an increase in pH under the weakly acidic conditions. Selective adsorption with the local maximum at the Er ion and local minimum at the Er
ion and local minimum at the Er ion was observed, similar to the selective adsorption of the bacterial cell surface but different from that of orthophosphates. These results indicate that the adsorption of lanthanide on the phospholipid does not result from simple adsorption on orthophosphate functional groups but by the composition and molecular structure of the phospholipid. Our results strongly suggest that liposomes can be used as a simple biomembrane model without any biological activity for the study of adsorption of lanthanide ions.
ion was observed, similar to the selective adsorption of the bacterial cell surface but different from that of orthophosphates. These results indicate that the adsorption of lanthanide on the phospholipid does not result from simple adsorption on orthophosphate functional groups but by the composition and molecular structure of the phospholipid. Our results strongly suggest that liposomes can be used as a simple biomembrane model without any biological activity for the study of adsorption of lanthanide ions.
 fungal cells; A pH-dependent contribution of phosphoryl functional group
fungal cells; A pH-dependent contribution of phosphoryl functional groupJiang, M. Y.*; Onuki, Toshihiko; Yamasaki, Shinya; Tanaka, Kazuya*; Utsunomiya, Satoshi*
Journal of Radioanalytical and Nuclear Chemistry, 295(3), p.2283 - 2287, 2013/03
Times Cited Count:3 Percentile:25.73(Chemistry, Analytical)The adsorption of Ytterbium on the cells of yeast  has been studied by batch type experiment by changing solution pH. The Yb adsorption species on the yeast cell wall of the
has been studied by batch type experiment by changing solution pH. The Yb adsorption species on the yeast cell wall of the  was determined by extended X-ray absorption fine structure (EXAFS) spectroscopy combined with a linear combination analysis at various pHs. The results indicated that the contribution of Yb-phosphoryl species was constant between pH 3 and 5, strongly suggesting that most of the Yb was associated with undeprotonated phosphoryl functional groups.
was determined by extended X-ray absorption fine structure (EXAFS) spectroscopy combined with a linear combination analysis at various pHs. The results indicated that the contribution of Yb-phosphoryl species was constant between pH 3 and 5, strongly suggesting that most of the Yb was associated with undeprotonated phosphoryl functional groups.
Kozai, Naofumi; Onuki, Toshihiko; Arisaka, Makoto; Watanabe, Masayuki; Sakamoto, Fuminori; Yamasaki, Shinya; Jiang, M.
Journal of Nuclear Science and Technology, 49(5), p.473 - 478, 2012/05
Times Cited Count:64 Percentile:97.82(Nuclear Science & Technology)Chemical states of radioactive Cs in the contaminated soils by Fukushima Daiichi Nuclear Power Plant accident has been characterized by the desorption experiments using appropriate reagents solutions and size fractionation of the contaminated soils. Approximately 70% of radioactive Cs in the residual fraction were associated with the size fractions larger than the elutriated one, even though mica-like minerals were contained in the elutriated one. These results strongly suggest that radioactive Cs was irreversibly associated with soil components other than mica like minerals in the contaminated soil.