Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Oya, Yasuhisa*; Li, X.*; Sato, Misaki*; Yuyama, Kenta*; Oyaizu, Makoto; Hayashi, Takumi; Yamanishi, Toshihiko; Okuno, Kenji*
Journal of Nuclear Science and Technology, 53(3), p.402 - 405, 2016/03
Times Cited Count:10 Percentile:68.36(Nuclear Science & Technology)The deuterium (D) permeation behaviors for ion damaged tungsten (W) by 3 keV D
 and 10 keV C
and 10 keV C were studied. The D permeability was obtained for un-damaged W at various temperatures. For both D
were studied. The D permeability was obtained for un-damaged W at various temperatures. For both D
 and C
and C implanted W, the permeability was clearly reduced. But, for the D
implanted W, the permeability was clearly reduced. But, for the D
 implanted W, the permeability was recovered by heating at 1173 K and it was almost consistent with that for un-damaged W. In the case of C
implanted W, the permeability was recovered by heating at 1173 K and it was almost consistent with that for un-damaged W. In the case of C implanted W, the permeability was not recovered even if the sample was heated at 1173 K, indicating that the existence of carbon would prevent the recovery of permeation path in W. In addition, TEM observation showed the voids were grown by heating at 1173 K and not removed, showing the existence of damages would not largely influence on the hydrogen permeation behavior in W in the present study.
implanted W, the permeability was not recovered even if the sample was heated at 1173 K, indicating that the existence of carbon would prevent the recovery of permeation path in W. In addition, TEM observation showed the voids were grown by heating at 1173 K and not removed, showing the existence of damages would not largely influence on the hydrogen permeation behavior in W in the present study.
Yamanishi, Toshihiko
Purazuma, Kaku Yugo Gakkai-Shi, 92(1), p.21 - 25, 2016/01
In a fusion reactor, the hydrogen isotope separation system is required in the fuel cycle system to supply deuterium (D) and tritium (T) as its fuel. In ITER, 90% of T must be recycled through the isotope separation system. On the other hand; since the hydrogen (H) gas is finally exhausted to the environment, the T concentration in the H gas from the isotope separation system should be as low as reasonable achievable. Hence, the isotope separation system of a fusion reactor must have a large separation factor. The flow rate of the isotope separation system of a fusion reactor reaches to 300 mol/h. Only the cryogenic distillation method can meet the above conditions (large flow rate and separation factor) and is most likely used as a hydrogen separation system in a fusion reactor. In this chapter, several simulation methods and a set of experimental data of the cryogenic distillation columns are described in detail.
Isobe, Kanetsugu; Kawamura, Yoshinori; Iwai, Yasunori; Oyaizu, Makoto; Nakamura, Hirofumi; Suzuki, Takumi; Yamada, Masayuki; Edao, Yuki; Kurata, Rie; Hayashi, Takumi; et al.
Fusion Engineering and Design, 98-99, p.1792 - 1795, 2015/10
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)Activities on Broader Approach (BA) were started in 2007 on the basis of the Agreement between the Government of Japan and the EURATOM. The period of BA activities consist of Phase1 and Phase2 dividing into Phase 2-1 (2010-2011), Phase 2-2 (2012-2013) and Phase 2-3 (2014-2016). Tritium technology was chosen as one of important R&D issues to develop DEMO plant. R&D activities of tritium technology on BA consist of four tasks. Task-1 is to prepare and maintain the tritium handling facility in Rokkasho BA site in Japan. Task 2, 3 and 4 are main R&D activities for tritium and these are focused on: Task-2) Development of tritium accountancy technology, Task-3) Development of basic tritium safety research, Task-4) Tritium durability test. R&D activities of tritium technology in Phase 2-2 were underway successfully and closed in 2013.
Hayashi, Takumi; Nakamura, Hirofumi; Kawamura, Yoshinori; Iwai, Yasunori; Isobe, Kanetsugu; Yamada, Masayuki; Suzuki, Takumi; Kurata, Rie; Oyaizu, Makoto; Edao, Yuki; et al.
Fusion Science and Technology, 67(2), p.365 - 370, 2015/03
Times Cited Count:1 Percentile:9.74(Nuclear Science & Technology)Edao, Yuki; Kawamura, Yoshinori; Kurata, Rie; Fukada, Satoshi*; Takeishi, Toshiharu*; Hayashi, Takumi; Yamanishi, Toshihiko
Fusion Science and Technology, 67(2), p.320 - 323, 2015/03
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)The present study aims at obtaining fundamental knowledge for tritium transfer behavior and interaction between tritium and paint coated on concrete walls. The amounts of tritium penetration and release in cement paste with epoxy and urethane paint coatings were measured. The tritium penetration amounts were increased with the HTO exposure time. Time to achieve each saturate tritium value was more than 60 days for cement paste coated with epoxy paint and with urethane paint, while cement paste without paint took 2 days to achieve it. Tritium penetration rates were estimated by an analysis of diffusion model. Although their paint coatings were effective for reduction of tritium penetration through the cement paste exposed to HTO for a short period, the amount of tritium trapped in the paints became large for a long time. This work has been performed under the collaboration research between JAEA and Kyushu University.
Fukada, Satoshi*; Katayama, Kazunari*; Takeishi, Toshiharu*; Edao, Yuki; Kawamura, Yoshinori; Hayashi, Takumi; Yamanishi, Toshihiko
Fusion Science and Technology, 67(2), p.99 - 102, 2015/03
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)Enoeda, Mikio; Tanigawa, Hisashi; Hirose, Takanori; Nakajima, Motoki; Sato, Satoshi; Ochiai, Kentaro; Konno, Chikara; Kawamura, Yoshinori; Hayashi, Takumi; Yamanishi, Toshihiko; et al.
Fusion Engineering and Design, 89(7-8), p.1131 - 1136, 2014/10
Times Cited Count:21 Percentile:84.18(Nuclear Science & Technology)The development of a Water Cooled Ceramic Breeder (WCCB) Test Blanket Module (TBM) is being performed as one of the most important steps toward DEMO blanket in Japan. Regarding the fabrication technology development using F82H, the fabrication of a real scale mockup of the back wall of TBM was completed. Also the assembling of the complete box structure of the TBM mockup and planning of the pressurization testing was studied. The development of advanced breeder and multiplier pebbles for higher chemical stability was performed for future DEMO blanket application. From the view point of TBM test result evaluation and DEMO blanket performance design, the development of the blanket tritium simulation technology, investigation of the TBM neutronics measurement technology and the evaluation of tritium production and recovery test using D-T neutron in the Fusion Neutronics Source (FNS) facility has been performed.
Iwai, Yasunori; Sato, Katsumi; Yamanishi, Toshihiko
Fusion Engineering and Design, 89(7-8), p.1534 - 1538, 2014/10
Times Cited Count:8 Percentile:53.31(Nuclear Science & Technology)The ion exchange membrane such as Nafion is a key material for electrolysis cells of the Water Detritiation System. Long-term exposure of Nafion ion exchange membrane into 1.38 10
10 Bq/kg of tritiated water was conducted at room temperature for up to 2 years. The ionic conductivity of Nafion ion exchange membrane after immersed in tritiated water was changed. The change in color of membrane from colorless to yellowish was caused by active radical reactions. Infrared Fourier transform spectrum of the membrane immersed in tritiated water revealed a small peak for bending vibration of C-H situated at 1437 cm
Bq/kg of tritiated water was conducted at room temperature for up to 2 years. The ionic conductivity of Nafion ion exchange membrane after immersed in tritiated water was changed. The change in color of membrane from colorless to yellowish was caused by active radical reactions. Infrared Fourier transform spectrum of the membrane immersed in tritiated water revealed a small peak for bending vibration of C-H situated at 1437 cm demonstrating the formation of hydrophobic functional group in the membrane. The high-resolution solid state
demonstrating the formation of hydrophobic functional group in the membrane. The high-resolution solid state  F NMR spectrum of the membrane after immersed in tritiated water was similar to that of membrane irradiated with
F NMR spectrum of the membrane after immersed in tritiated water was similar to that of membrane irradiated with  -rays. From the
-rays. From the  F NMR spectrum, any distinctive degradation in the membrane structure by interaction with tritium was not measured.
F NMR spectrum, any distinctive degradation in the membrane structure by interaction with tritium was not measured.
Edao, Yuki; Kawamura, Yoshinori; Yamanishi, Toshihiko; Fukada, Satoshi*
Fusion Engineering and Design, 89(9-10), p.2062 - 2065, 2014/10
Times Cited Count:4 Percentile:30.92(Nuclear Science & Technology)Tritium transfer behavior through hydrophobic paints, epoxy resin and acrylic-silicon resin, was investigated experimentally. The authors measured the amount of tritium permeated through the paint membranes which exposed in HTO atmosphere of 2 100 Bq/cm
100 Bq/cm . The most of tritium permeated through the paints in the form of HTO at room temperature. Tritium permeation through the acrylic-silicon paint was explained a linear sorption/release model and that through the epoxy paint was suggested to be controlled by a one-dimensional diffusion model. While effective diffusivity was 1.0
. The most of tritium permeated through the paints in the form of HTO at room temperature. Tritium permeation through the acrylic-silicon paint was explained a linear sorption/release model and that through the epoxy paint was suggested to be controlled by a one-dimensional diffusion model. While effective diffusivity was 1.0 10
10
 1.8
1.8 10
10 m
m /s at 21
/s at 21 C
C 26
26 C for epoxy membrane, the diffusivity was found to be hundreds times larger than that for cement-paste coated with epoxy paint. Hence, tritium diffusivity through interface between cement-paste and the epoxy paint was considered to be most effective in the overall tritium transfer process. Tritium transfer behavior in the interface is important to explain the mechanism of tritium transfer behavior in concrete walls.
C for epoxy membrane, the diffusivity was found to be hundreds times larger than that for cement-paste coated with epoxy paint. Hence, tritium diffusivity through interface between cement-paste and the epoxy paint was considered to be most effective in the overall tritium transfer process. Tritium transfer behavior in the interface is important to explain the mechanism of tritium transfer behavior in concrete walls.
Kawamura, Yoshinori; Edao, Yuki; Iwai, Yasunori; Hayashi, Takumi; Yamanishi, Toshihiko
Fusion Engineering and Design, 89(7-8), p.1539 - 1543, 2014/10
Times Cited Count:8 Percentile:53.31(Nuclear Science & Technology)Tritium recovery system using adsorption or catalytic isotope exchange has already been proposed for a solid breeding blanket system of a nuclear fusion reactor. Synthetic zeolite is often used as an adsorbent or a substrate of chemical exchange catalyst. And, it is well known that its properties are changed easily by exchanging their cations. So, in this work, adsorption capacities of hydrogen isotope and water vapor on cation-exchanged mordenite with transition metal ion were investigated. Ag ion-exchanged mordenite (Ag-MOR) has indicated considerably large hydrogen adsorption capacity in lower pressure range at 77 K. And, adsorption capacity of water vapor did not so vary with exchaned cation in comparison with hydrogen adsorption. The discussion from the viewpoint of adsorption rate is still remaining, but more compact cryosorption column for tritium recovery system is possible to design if Ag-MOR is adopted.
Hayashi, Takumi; Isobe, Kanetsugu; Nakamura, Hirofumi; Kobayashi, Kazuhiro; Oya, Yasuhisa*; Okuno, Kenji*; Oyaizu, Makoto; Edao, Yuki; Yamanishi, Toshihiko
Fusion Engineering and Design, 89(7-8), p.1520 - 1523, 2014/10
Times Cited Count:1 Percentile:8.88(Nuclear Science & Technology)Tritium confinement is the most important safety issue in the fusion reactor. Tritium behavior on the water metal boundary is very important to design tritium plant with breading blanket system using cooling water. A series of tritium permeation experiment into pressurized water or water vapor jacket with He or Ar have been performed through pure iron piping with/without 7 micro-meter gold plating, which contained about 1 kPa of pure tritium gas at 423 K, with monitoring the chemical forms of tritium. Also, deuterium permeation experiments from heavy water vessel through various metal piping, such as pure iron (Fe), nickel (Ni), stainless steel (SS304), and pure iron with 10 micro-meter gold plating, were performed at 573 K and at 15 MPa. Recently, using the above heavy water system, we have succeeded to detect simultaneous hydrogen isotopes transfer from and to the metal surface by introducing H gas to the metal piping after stabilized deuterium permeation was detected.
gas to the metal piping after stabilized deuterium permeation was detected.
Iwai, Yasunori; Sato, Katsumi; Yamanishi, Toshihiko
Fusion Science and Technology, 66(1), p.214 - 220, 2014/07
Times Cited Count:4 Percentile:30.92(Nuclear Science & Technology)We have developed a honeycomb Pd catalyst applicable for the oxidation of the tritiated hydrocarbons. In this study, honeycomb Pd catalysts of three different densities, 2, 5 and 10 g/L, were prepared to investigate the effect of density on reaction rate. Tritiated methane was selected as a typical hydrocarbon. Overall reaction rate constant for tritiated methane oxidation on honeycomb Pd catalyst were determined with a flow-through system as a function of space velocity from 1000 to 6300 h , methane concentration in carrier from 0.004 to 100 ppm, temperature of catalyst from 322 to 673 K. The density of palladium deposited on the base material had little effect on reaction rate for tritiated methane oxidation. The overall reaction rate constant was proportional to the space velocity. The overall reaction rate constant was independent on the methane concentration when it was less than 10 ppm.
, methane concentration in carrier from 0.004 to 100 ppm, temperature of catalyst from 322 to 673 K. The density of palladium deposited on the base material had little effect on reaction rate for tritiated methane oxidation. The overall reaction rate constant was proportional to the space velocity. The overall reaction rate constant was independent on the methane concentration when it was less than 10 ppm.
Kawamura, Yoshinori; Iwai, Yasunori; Munakata, Kenzo*; Yamanishi, Toshihiko
Journal of Nuclear Materials, 442(1-3), p.S455 - S460, 2013/11
Times Cited Count:13 Percentile:69.61(Materials Science, Multidisciplinary)Zeolite easily exchanges its cation to another. In this work, synthetic mordenite type zeolite (Na-MOR) was used as start material. And, its cation (Na ) has been exchanged by Li
) has been exchanged by Li , K
, K , Mg
, Mg and Ca
and Ca . Then, adsorption capacities of H
. Then, adsorption capacities of H and D
and D on them were investigated at 77 K, 159 K, 175 K and 195 K. Adsorption capacities on Li-MOR and Ca-MOR became larger than that on Na-MOR at low pressure range. Oppositely, that on K-MOR became smaller. In case of alkaline metal, cation with small atomic number may lead to large adsorption capacity.
on them were investigated at 77 K, 159 K, 175 K and 195 K. Adsorption capacities on Li-MOR and Ca-MOR became larger than that on Na-MOR at low pressure range. Oppositely, that on K-MOR became smaller. In case of alkaline metal, cation with small atomic number may lead to large adsorption capacity.
Iwai, Yasunori; Sato, Katsumi; Kawamura, Yoshinori; Yamanishi, Toshihiko
Fusion Engineering and Design, 88(9-10), p.2319 - 2322, 2013/10
Times Cited Count:4 Percentile:32.48(Nuclear Science & Technology)The Nafion ion exchange membrane is a key material for electrolysis cells of the water detritiation system. Endurance of ion exchange membrane immersed into high-concentration tritiated water has been demonstrated under the Broader Approach activities, as a R&D on endurance of fuel cycle components at high tritium exposure. Long-term exposure of Nafion ion exchange membrane into 1.38 TBq/kg of tritiated water was conducted at room temperature for up to 2 years. The curves of percent elongation at break vs. dose and tensile strength vs. dose for the Nafion membranes immersed into tritiated water were well consistent with those for Nafion membranes irradiated to an equivalent dose with  rays and electron beams. The results of ferric Fenton test indicated that the degradation directly by radiation was dominant at room temperature compared with that by reactions with radicals produced from water radiolysis. The curve of ion exchange capacity vs. dose for the Nafion membranes immersed into tritiated water was also well consistent with that for Nafion membranes irradiated to an equivalent dose with
rays and electron beams. The results of ferric Fenton test indicated that the degradation directly by radiation was dominant at room temperature compared with that by reactions with radicals produced from water radiolysis. The curve of ion exchange capacity vs. dose for the Nafion membranes immersed into tritiated water was also well consistent with that for Nafion membranes irradiated to an equivalent dose with  rays and electron beams. These results showed that the irradiation tests with
rays and electron beams. These results showed that the irradiation tests with  rays and electron beams were effective to predict a degradation behavior of ion exchange membrane immersed into high-concentration tritiated water.
rays and electron beams were effective to predict a degradation behavior of ion exchange membrane immersed into high-concentration tritiated water.
Yamanishi, Toshihiko; Kawamura, Yoshinori; Iwai, Yasunori; Isobe, Kanetsugu
Fusion Engineering and Design, 88(9-10), p.2272 - 2275, 2013/10
Times Cited Count:2 Percentile:18.63(Nuclear Science & Technology)The multi-purpose RI equipment has been constructed at Rokkasho site in DEMO R&D building until 2011. The equipment is the first and unique facility in Japan, where tritium, RI species, and beryllium can simultaneously be used. The amounts of tritium used and stored are 3.7 TBq per day and 7.4 TBq, respectively. The material of the column of the micro gas chromatograph has been studied. The calorimeter has also been studied as a possible tritium measurement method. A set of basic data on the interaction between materials and tritium has been measured especially for pure Fe. As for the tritium behavior in the blanket materials, the tritium release after neutron irradiation was studied. As a study for the tritium durability, the endurance of ion exchange membrane has been tested by using high concentration tritium water. The data of tritium water were well consistent with those obtained by  irradiation.
irradiation.
Kawamura, Yoshinori; Edao, Yuki; Yamanishi, Toshihiko
Fusion Engineering and Design, 88(9-10), p.2255 - 2258, 2013/10
Times Cited Count:8 Percentile:53.52(Nuclear Science & Technology)To develop an adsorbent that is suitable for a separation column of gas chromatograph for hydrogen isotope analysis, the mordenite-type zeolite of which cations (Na ) were exchanged with other cations have been prepared and their hydrogen isotope adsorption behavior is being investigated. Then, it has been shown experimentally that mordenite-type zeolite of which cation has been exchanged with Ca
) were exchanged with other cations have been prepared and their hydrogen isotope adsorption behavior is being investigated. Then, it has been shown experimentally that mordenite-type zeolite of which cation has been exchanged with Ca (Ca-MOR) has fairly large adsorption capacity. So, breakthrough curves of H
(Ca-MOR) has fairly large adsorption capacity. So, breakthrough curves of H (or D
(or D ) adsorption on Ca-MOR at 194 K and 175 K have been observed and mass transfer coefficients have been estimated from them. The rate-controlling step of hydrogen adsorption is hydrogen diffusion in porous adsorbent. And, isotopic difference of effective diffusivity in Ca-MOR is larger than that in Na-MOR. Therefore, in comparison with Na-MOR, use of Ca-MOR is expected to enhance the hydrogen isotope separation capability.
) adsorption on Ca-MOR at 194 K and 175 K have been observed and mass transfer coefficients have been estimated from them. The rate-controlling step of hydrogen adsorption is hydrogen diffusion in porous adsorbent. And, isotopic difference of effective diffusivity in Ca-MOR is larger than that in Na-MOR. Therefore, in comparison with Na-MOR, use of Ca-MOR is expected to enhance the hydrogen isotope separation capability.
 TiO
TiO under D-T neutron irradiation
under D-T neutron irradiationEdao, Yuki; Kawamura, Yoshinori; Ochiai, Kentaro; Hoshino, Tsuyoshi; Takakura, Kosuke; Ota, Masayuki; Iwai, Yasunori; Yamanishi, Toshihiko; Konno, Chikara
JAEA-Research 2012-040, 15 Pages, 2013/02
Tritium generation and recovery studies on Li TiO
TiO as a solid breeding material under neutron irradiation carried out in the Fusion Neutron Source (FNS) facility. A capsule with Li
as a solid breeding material under neutron irradiation carried out in the Fusion Neutron Source (FNS) facility. A capsule with Li TiO
TiO packed bed was put in a system which simulated an actual blanket system which built in beryllium blocks and lithium titanate ones. Estimated values of the amount of tritium generation by a numerical calculation agreed closely with experimental values. The capsule was heated up to 300
packed bed was put in a system which simulated an actual blanket system which built in beryllium blocks and lithium titanate ones. Estimated values of the amount of tritium generation by a numerical calculation agreed closely with experimental values. The capsule was heated up to 300 C, and helium, helium with water vapor, hydrogen or hydrogen/water vapor were selected as purge gas. In the case of purge by helium added water vapor, the ratio of HTO to total tritium release was 98%. In helium with hydrogen/water vapor purge, the ratio of HTO to total tritium release was 80%, which was confirmed that HTO released by isotope exchange reaction between water vapor and tritium. In helium with hydrogen purge, the ratio of HT to total tritium release was 60
C, and helium, helium with water vapor, hydrogen or hydrogen/water vapor were selected as purge gas. In the case of purge by helium added water vapor, the ratio of HTO to total tritium release was 98%. In helium with hydrogen/water vapor purge, the ratio of HTO to total tritium release was 80%, which was confirmed that HTO released by isotope exchange reaction between water vapor and tritium. In helium with hydrogen purge, the ratio of HT to total tritium release was 60 70%, which was shown that HT released by isotope exchange reaction between hydrogen gas and tritium. HTO released by water generation reaction between hydrogen in purge gas and oxygen in Li
70%, which was shown that HT released by isotope exchange reaction between hydrogen gas and tritium. HTO released by water generation reaction between hydrogen in purge gas and oxygen in Li TiO
TiO although water vapor was not added in purge gas. The ratio of HTO release seemed to be small under the deoxidized condition of the Li
although water vapor was not added in purge gas. The ratio of HTO release seemed to be small under the deoxidized condition of the Li TiO
TiO surface. Tritium release behavior in the Li
surface. Tritium release behavior in the Li TiO
TiO depended on the composition of purge gas, and its chemical form was affected by the surface conditions of Li
depended on the composition of purge gas, and its chemical form was affected by the surface conditions of Li TiO
TiO .
.
Isobe, Kanetsugu; Alimov, V. Kh.*; Taguchi, Akira*; Saito, Makiko; Torikai, Yuji*; Hatano, Yuji*; Yamanishi, Toshihiko
Journal of Plasma and Fusion Research SERIES, Vol.10, p.81 - 84, 2013/02
The distribution of hydrogen trapping sites on W surface exposed with D plasma was examined by the techniques of imaging plate and autoradiography. Recrystallized W specimens were exposed with D plasma at around 495 and 550 K to the same fluence of 10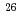 D/m
D/m . Then, tritium was introduced into specimen by the exposure to tritium gaseous at 473 K. After that, the tritium distribution on W surface was examined by the techniques of imaging plate and autoradiography. From the results of the imaging plate, tritium was found to be highly concentrated within the area exposed with D plasma and the concentration of tritium was slightly varied even in that area. In the autoradiograph of W surface, it was found that tritium concentrated on the grain boundary and blisters.
. Then, tritium was introduced into specimen by the exposure to tritium gaseous at 473 K. After that, the tritium distribution on W surface was examined by the techniques of imaging plate and autoradiography. From the results of the imaging plate, tritium was found to be highly concentrated within the area exposed with D plasma and the concentration of tritium was slightly varied even in that area. In the autoradiograph of W surface, it was found that tritium concentrated on the grain boundary and blisters.
Oyaizu, Makoto; Isobe, Kanetsugu; Yamanishi, Toshihiko
ECS Transactions, 50(50), p.63 - 69, 2013/00
Times Cited Count:3 Percentile:80.86(Electrochemistry)The effects of tritiated water on the passivation behavior of SUS304 stainless steel were electrochemically studied by anodic polarization measurements and diachronic measurements of open circuit potential with changing tritium concentration and dissolved oxygen concentration as parameters in the electrolyte of 1N sulfuric acid solution, where the passivation inhibitory effects by tritiated water could be clearly observed. As a result, it was found that the passivation would be proceed with two steps. The effects of tritiated water could be observed in both of two steps; delay in the first step and deceleration in the second step. From these results, it was suggested that the passivation inhibitory effect might be promoted by further oxidation and sequential dissolution of Cr by radiolysis products.
by radiolysis products.
Enoeda, Mikio; Tanigawa, Hisashi; Hirose, Takanori; Suzuki, Satoshi; Ochiai, Kentaro; Konno, Chikara; Kawamura, Yoshinori; Yamanishi, Toshihiko; Hoshino, Tsuyoshi; Nakamichi, Masaru; et al.
Fusion Engineering and Design, 87(7-8), p.1363 - 1369, 2012/08
Times Cited Count:35 Percentile:92.09(Nuclear Science & Technology)The development of a Water Cooled Ceramic Breeder (WCCB) Test Blanket Module (TBM) is being performed as one of the most important steps toward DEMO blanket in Japan. For the TBM testing and evaluation toward DEMO blanket, the module fabrication technology development by a candidate structural material, reduced activation martensitic/ferritic steel, F82H, is one of the most critical items from the viewpoint of realization of TBM testing in ITER. Fabrication of a real scale first wall, side walls, a breeder pebble bed box and assembling of the first wall and side walls have succeeded. Recently, the real scale partial mockup of the back wall was fabricated. The fabrication procedure of the back wall, whose thickness is up to 90 mm, was confirmed toward the fabrication of the real scale back wall by F82H. This paper overviews the recent achievements of the development of the WCCB TBM in Japan.