Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ishidera, Takamitsu; Okazaki, Mitsuhiro*; Yamada, Yoshihide*; Tomura, Tsutomu*; Shibutani, Sanae*
Journal of Nuclear Science and Technology, 60(5), p.536 - 546, 2023/05
The distribution coefficient ( ) value of radionuclides is an important parameter in the radionuclide migration analysis in the safety assessment of the geological disposal of high-level radioactive waste. The
) value of radionuclides is an important parameter in the radionuclide migration analysis in the safety assessment of the geological disposal of high-level radioactive waste. The  values must be extensively evaluated especially under conditions where they might be decreased to improve the reliability of safety assessment. In this study, the pH dependence of the
values must be extensively evaluated especially under conditions where they might be decreased to improve the reliability of safety assessment. In this study, the pH dependence of the  values for Sn and Nb on montmorillonite was evaluated using batch sorption experiments at neutral to alkaline pH, which might be caused by the leaching of cementitious materials and the corrosion of carbon steel. The
values for Sn and Nb on montmorillonite was evaluated using batch sorption experiments at neutral to alkaline pH, which might be caused by the leaching of cementitious materials and the corrosion of carbon steel. The  values were determined in the range 8
values were determined in the range 8  pH
pH  12 by the experiments and were found to decrease with increasing pH. A model calculation using a thermodynamic sorption model was conducted on the measured pH dependence of the
12 by the experiments and were found to decrease with increasing pH. A model calculation using a thermodynamic sorption model was conducted on the measured pH dependence of the  values. Two different sorption sites were required to describe the pH dependence of the
values. Two different sorption sites were required to describe the pH dependence of the  values of Sn in the model calculation, whereas one sorption site was considered predominant in the sorption of Nb.
values of Sn in the model calculation, whereas one sorption site was considered predominant in the sorption of Nb.
Kitamura, Akira; *
JNC TN8400 2001-009, 54 Pages, 2001/01
Spectroscopic measurements of neodymium(III) and samarium(III) were carried out by spectrophotometer and laser-induced photoacoustic spectroscopic (LPAS) system for the investigation of the detection limit of both systems. The absorption spectra and photoacoustic spectra of Nd and Sm
and Sm were obtained with varying the concentration of the ions from 2
were obtained with varying the concentration of the ions from 2 10
10 to 2
to 2 10
10 mol
mol dm
dm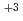 . The absorption spectrum of Nd
. The absorption spectrum of Nd was also determined by a special spectrophotometer, of which the measurement cell was set in a glove box filled with inert nitrogen gas. For the comparison with these photoacoustic and absorption spectra, the absorption spectra of Nd
was also determined by a special spectrophotometer, of which the measurement cell was set in a glove box filled with inert nitrogen gas. For the comparison with these photoacoustic and absorption spectra, the absorption spectra of Nd and Sm
and Sm were determined by an usual spectrophotometer with the light-path lengths of 1 cm and 10 cm. The detection limit of the photoacoustic measurement was reported much lower than that of absorbance measurement by several researchers. However, the present study was concluded that the detection limit of photoacoustic measurement with the present LPAS system was similar to that of absorbance measurement with the light-path length of 10 cm. The detection limits of neptunium(IV,V) were estimated and the possibility of the speciation of neptunium(IV) was discussed from the results of the present study.
were determined by an usual spectrophotometer with the light-path lengths of 1 cm and 10 cm. The detection limit of the photoacoustic measurement was reported much lower than that of absorbance measurement by several researchers. However, the present study was concluded that the detection limit of photoacoustic measurement with the present LPAS system was similar to that of absorbance measurement with the light-path length of 10 cm. The detection limits of neptunium(IV,V) were estimated and the possibility of the speciation of neptunium(IV) was discussed from the results of the present study.
; ; Kuroha, Mitsuhiko; Yui, Mikazu; *; *
JAERI-Conf 99-004, p.643 - 653, 1999/03
None
Kuroha, Mitsuhiko; ; Yamada, Kazuo; Yui, Mikazu; *; *
JNC TN8410 98-001, 35 Pages, 1998/10
In order to study the migration behavior of plutonium from high-level vitrified waste in a deep repository condition, plutonium doped simulated high-level vitrified waste was prepared in a laboratory. Pu-doped glass was prepared with 1wt.% of PuO . The simulated high-level vitrified waste was crushed to less than 75
. The simulated high-level vitrified waste was crushed to less than 75 m and put into platinum crucible (
m and put into platinum crucible (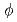 17 mm
17 mm  80 mm) with PuO
80 mm) with PuO powder sieved to less than 75
powder sieved to less than 75 m. The crucible was set into an electric furnace and the temperature was raised up to 1300
m. The crucible was set into an electric furnace and the temperature was raised up to 1300  C and kept for 2 hours. The melt was cooled down after annealing at 650
C and kept for 2 hours. The melt was cooled down after annealing at 650  C for 2 hours. The specimen was cut and homogeneity of Pu in Pu-doped glass was observed by
C for 2 hours. The specimen was cut and homogeneity of Pu in Pu-doped glass was observed by  -autoradiography. As a result of observation, plutonium was distributed homogeneously in the middle of the glass, but was distributed heterogeneously in the bottom of glass. And then the whole of the glass was crushed and melted again in the same way. Leaching experiments of Pu-doped glass and solubility experiments of Pu were carried out under aerobic conditions at room temperature. Leaching experiments of Pu-doped glass were carried out by using batch method with NaNO
-autoradiography. As a result of observation, plutonium was distributed homogeneously in the middle of the glass, but was distributed heterogeneously in the bottom of glass. And then the whole of the glass was crushed and melted again in the same way. Leaching experiments of Pu-doped glass and solubility experiments of Pu were carried out under aerobic conditions at room temperature. Leaching experiments of Pu-doped glass were carried out by using batch method with NaNO solution. The particle size of glass powder was under 75
solution. The particle size of glass powder was under 75 m and pH were adjusted by HNO
m and pH were adjusted by HNO and NaOH in the pH range 4-10. The experimental period was 151-340 days. Solubility experiments of Pu were also carried out from over saturation in 0.1M NaNO
and NaOH in the pH range 4-10. The experimental period was 151-340 days. Solubility experiments of Pu were also carried out from over saturation in 0.1M NaNO solution and pH were adjusted by HNO
solution and pH were adjusted by HNO and NaOH in the pH range 4-12. The experimental period was 42-233 days. These leachate were filtered through a 10,000 molecular weight cut off (MWCO) ultrafilter to separate solution and solid. These experiments were carried out in the teflon vessel. The Pu concentration in leachate was analyzed by
and NaOH in the pH range 4-12. The experimental period was 42-233 days. These leachate were filtered through a 10,000 molecular weight cut off (MWCO) ultrafilter to separate solution and solid. These experiments were carried out in the teflon vessel. The Pu concentration in leachate was analyzed by  -spectrometry. The Pu concentration from the Pu-doped glass was around 10
-spectrometry. The Pu concentration from the Pu-doped glass was around 10 mol/l in the pH range 4-6, and decreased to 10
mol/l in the pH range 4-6, and decreased to 10 mol/l with increasing pH. The leaching ...
mol/l with increasing pH. The leaching ...
; *; *; Tanimoto, Kenichi; Enokido, Yuji
PNC TN9410 92-208, 68 Pages, 1992/07
The plan of the gelogic disposal of high level waste that need to do estimate the effect of radiation in near field surrouding waste. We have applied "JOYO" spent fuel storage pool as irradiation field and investigated the effect of gamma radiation about quality of groundwater, because we have to obtained a basic data related geologic dispose under irradiaton condition. The experiment was applied artificial brine for simulated groundwater. The same samples were located in "JOYO" spent fuel storage pool without gamma radiation and other effects were estimated. The samples were also observed the variation of quality of artifical brine every fixed time after irradiation and were estimated the effect as the function of time, gamma irradiation were carried out from 24hours (1.0 10
10
 1.3
1.3 10
10 Gy) to 1440 hours (4.4
Gy) to 1440 hours (4.4 10
10
 6.8
6.8 10
10 Gy). The results indicate the following. (1)The change of pH, conductivity and ion concentrations in artifical brine could not be observed in the samples before and after irradiation. (2)Eh of the samples was 241mV before irradiation, but it decreased 156mV after irradiaton for 1440 hours. Eh tend to decrease by increase of the absorption dose. (3)Do of the samples before and after irradiation for 1440 hours were 20.76 and 5930
Gy). The results indicate the following. (1)The change of pH, conductivity and ion concentrations in artifical brine could not be observed in the samples before and after irradiation. (2)Eh of the samples was 241mV before irradiation, but it decreased 156mV after irradiaton for 1440 hours. Eh tend to decrease by increase of the absorption dose. (3)Do of the samples before and after irradiation for 1440 hours were 20.76 and 5930  g/
g/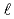 , respectively. Do tend to increases by increase of the absorption dose. (4)Before and after irradiation test for 480 hours, nitric ion was detected 2.9 and 105ppm, respectively, In no gamma irradiaton test, nitric ion was detdcted 4.0 and 5.6ppm, respectively. For 1440 hours, nitric ion was detected 15ppm after irradiation and 11ppm after rest without gamma irradiation. (5)pH, Eh, Do, conductivity and all ion concentrations in artifical brine have no the variation as the function ot time within fixed time (about 4hours) after irradiation. These results suggest that oxygen which were generated by the gamma radiolysis of water was incrased Do, ...
, respectively. Do tend to increases by increase of the absorption dose. (4)Before and after irradiation test for 480 hours, nitric ion was detected 2.9 and 105ppm, respectively, In no gamma irradiaton test, nitric ion was detdcted 4.0 and 5.6ppm, respectively. For 1440 hours, nitric ion was detected 15ppm after irradiation and 11ppm after rest without gamma irradiation. (5)pH, Eh, Do, conductivity and all ion concentrations in artifical brine have no the variation as the function ot time within fixed time (about 4hours) after irradiation. These results suggest that oxygen which were generated by the gamma radiolysis of water was incrased Do, ...
Fujiwara, Kenso; Kohara, Yukitoshi*; Okazaki, Mitsuhiro*; Suzuki, Yasuyuki*
no journal, ,
The stability constants of Np(IV) with Ca and Si are measured by solubility method. In the case of coexistence of Ca or Si under high pH region, the solubility of tetravalent actinide was higher than the case of without Ca or Si in a recent study. However, in the case of Np(IV), the experiment was not measured. Therefore, the solubility of Np(IV) was measured with Ca or Si in the high pH region, the stability constants of (Ca Np(OH)
Np(OH)
 ) and Si of Ca and the Np and complex (Np(OH)nSi
) and Si of Ca and the Np and complex (Np(OH)nSi O
O (OH)
(OH) etc) was calculated by solubility of Np(IV).
etc) was calculated by solubility of Np(IV).
Ishii, Yasuo; Seida, Yoshimi*; Tachi, Yukio; Okazaki, Mitsuhiro*; Kurosawa, Seiichi*
no journal, ,
We developed the Sorption/diffusion data acquisition method for high sorbing nuclides using trivalent actinide Am.
Terashima, Motoki; Seida, Yoshimi*; Okazaki, Mitsuhiro; Iwatsuki, Teruki; Iijima, Kazuki; Yui, Mikazu
no journal, ,
no abstracts in English
Ishii, Yasuo; Takahashi, Hiroaki; Seida, Yoshimi*; Tachi, Yukio; Nakazawa, Toshiyuki*; Kurosawa, Seiichi*; Okazaki, Mitsuhiro*
no journal, ,
We have developed Sorption/Diffusion Data Acquisition Method for High Sorbiong Am and Th in Horonobe Sedimentary Rock.
Ishii, Yasuo; Takahashi, Hiroaki; Tachi, Yukio; Tomura, Tsutomu*; Nemoto, Kazuaki*; Okazaki, Mitsuhiro*
no journal, ,
Am(III) diffusion experiment were performed by reservoir depletion (RD) test method coupled with thin layer ID profile fitting in 0.1 or 0.5M NaCl / 0.05M NaHCO - bentonite (kunipia-F) system. The Kd values were also measured using batch technique in the same experimental conditions. In an ordinary ID profile acquisition cutting the bentonite by scraper, the compacted bentonite sample can be cut into 100
- bentonite (kunipia-F) system. The Kd values were also measured using batch technique in the same experimental conditions. In an ordinary ID profile acquisition cutting the bentonite by scraper, the compacted bentonite sample can be cut into 100  m thick slices. Using this technique, it was possible to divide the ID profile into 10
m thick slices. Using this technique, it was possible to divide the ID profile into 10  m and therefore, to analyze diffusion distance layer larger than 50
m and therefore, to analyze diffusion distance layer larger than 50  m.
m.
Terashima, Motoki; Okazaki, Mitsuhiro; Iijima, Kazuki; Yui, Mikazu
no journal, ,
no abstracts in English
Terashima, Motoki; Okazaki, Mitsuhiro; Iijima, Kazuki; Yoshikawa, Hideki
no journal, ,
no abstracts in English
Ishii, Yasuo; Tomura, Tsutomu; Nemoto, Kazuaki; Okazaki, Mitsuhiro; Tachi, Yukio
no journal, ,
Am(III) diffusion experiment were performed by reservoir depletion (RD) test method coupled with thin layer ID profile fitting.
Terashima, Motoki; Saito, Takumi*; Okazaki, Mitsuhiro*; Tachi, Yukio; Iijima, Kazuki
no journal, ,
no abstracts in English
Sugiura, Yuki; Ishidera, Takamitsu; Suyama, Tadahiro*; Okazaki, Mitsuhiro*; Hamamoto, Takafumi*; Ishida, Keisuke*; Tachi, Yukio
no journal, ,
Ishidera, Takamitsu; Hamamoto, Takafumi*; Okazaki, Mitsuhiro*; Yamada, Yoshihide*; Tomura, Tsutomu*
no journal, ,
no abstracts in English