Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:22.61(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:2 Percentile:20.72(Chemistry, Inorganic & Nuclear)no abstracts in English
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:4 Percentile:12.17(Chemistry, Analytical)no abstracts in English
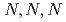 -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:13.73(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with 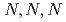 -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of 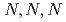 -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Metals, 8(7), p.558_1 - 558_10, 2018/07
Times Cited Count:13 Percentile:53.43(Materials Science, Multidisciplinary)The refining of platinum group metals is based mainly on solvent extraction methods, whereas Ru is selectively recovered by distillation as RuO . Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl
. Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl (H
(H O)
O) ]
] to [RuCl
to [RuCl ]
]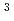
 with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru
with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru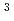
 had 5 Cl
had 5 Cl and 1 H
and 1 H O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl
O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl (H
(H O)]
O)]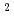
 on [RuCl
on [RuCl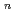 (H
(H O)
O)
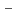
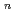 ]
]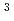

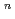 suggested that the EHBAA extracted the pentachlorido species.
suggested that the EHBAA extracted the pentachlorido species.
Narita, Hirokazu; Yaita, Tsuyoshi; Okamoto, Yoshihiro; Takai, Konomi; Shiwaku, Hideaki; Shimada, Asako*; Yamamoto, Takeshi*; Tanida, Hajime*
Spring-8 Experiment Report, Vol.6, P. 9, 2001/00
no abstracts in English
Okabe, Toshihiro*; Ono, Hiroyuki*; Hatayama, Ichiro*; Waguri, Atsushi*; Sawada, Yuzuru*; Tsugawa, Hidehito*; Ichida, Tadao*; Nakane, Akio*; Sano, Teruo*; Tanaka, Kazuaki*; et al.
no journal, ,
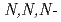 trimethylglycine
trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro
no journal, ,
no abstracts in English
小林 徹; 矢板 毅; 横山 啓一
尾形 剛志*; 成田 弘一*
【課題】希土類元素の相互分離を小規模、低コストで実現できる新規な希土類元素の吸着剤を提供する。 【解決手段】 希土類元素を含む溶液と接触して希土類元素を吸着する吸着剤であって、基材とフェナントロリン骨格を含み、前記基材にフェナントロリン骨格がアミド結合を介して導入されていることを特徴とする希土類元素の吸着剤。
小林 徹; 矢板 毅
松田 基史*; 大渡 啓介*; 尾形 剛志*; 成田 弘一*; 古郷 宏明*
【課題】希土類金属を抽出するための抽出剤として利用可能な新規化合物を提供することを課題とする。 【解決手段】下記一般式(1)で表されるフェナントロリン化合物又はその塩。