Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O
Sato, Tomonori; Kato, Chiaki; Ogi, Hirokazu*; Yamamoto, Masahiro; Ueno, Fumiyoshi
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2013 (USB Flash Drive), 6 Pages, 2013/10
The hydrogen peroxide (H O
O ) generated by the water radiolysis in the reactor coolant under irradiated condition plays an important role for the stress corrosion cracking (SCC) in the boiling water reactors (BWRs). In this study, the corrosive condition in high temperature water containing H
) generated by the water radiolysis in the reactor coolant under irradiated condition plays an important role for the stress corrosion cracking (SCC) in the boiling water reactors (BWRs). In this study, the corrosive condition in high temperature water containing H O
O was observed directly utilizing electrochemical impedance spectroscopy (EIS) in high temperature pure water. And the diffusion coefficient and thermal decomposition rate of H
was observed directly utilizing electrochemical impedance spectroscopy (EIS) in high temperature pure water. And the diffusion coefficient and thermal decomposition rate of H O
O at the 288
at the 288  C were estimated based on the obtained impedance responses. The reciprocal of the polarization resistance has linear relationship to the H
C were estimated based on the obtained impedance responses. The reciprocal of the polarization resistance has linear relationship to the H O
O concentration. The estimated diffusion coefficient of H
concentration. The estimated diffusion coefficient of H O
O (D
(D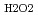 ) was 1.5
) was 1.5 10
10 cm
cm /s when the thickness of the diffusion layer was assumed to be 0.05 cm. The thermal decomposition rate of H
/s when the thickness of the diffusion layer was assumed to be 0.05 cm. The thermal decomposition rate of H O
O in 288
in 288 C was 0.042 s
C was 0.042 s .
.
Sato, Tomonori; Yamamoto, Masahiro; Kato, Chiaki; Tsukada, Takashi; Ogi, Hirokazu
Fushoku Boshoku Kyokai Dai-58-Kai Zairyo To Kankyo Toronkai Koenshu, p.185 - 188, 2011/09
The environmental factors for the corrosion of stainless steel were measured by the electrochemical impedance spectrometry in the high temperature pure water. And the analysis of the distribution of the flowing current between the electrodes was carried out to determine the cell factor for the impedance measurement system in this study and to determine the conductivity of the high temperature water more precisely. The current obtained theoretical analysis was 1.3 times larger than the current in the case that the cross section of the current path was equal to the area of the test specimen. The obtained conductivity relatively agreed with the literature data.