Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyazaki, Kanako*; Takehara, Masato*; Minomo, Kenta*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Saito, Takumi*; Onuki, Toshihiko*; Takano, Masahide; Shiotsu, Hiroyuki; et al.
Journal of Hazardous Materials, 470(15), p.134104_1 - 134104_11, 2024/05
Saito, Takumi*; Nishi, Shusaku*; Amano, Yuki; Beppu, Hikari*; Miyakawa, Kazuya
ACS ES&T Water (Internet), 3(12), p.4103 - 4112, 2023/12
Saito, Takumi*; Motokawa, Ryuhei; Okubo, Takahiro*; Miura, Daisuke*; Kumada, Takayuki
Environmental Science & Technology, 57(26), p.9802 - 9810, 2023/07
Times Cited Count:0 Percentile:0(Engineering, Environmental)Murota, Kento*; Aoyagi, Noboru; Mei, H.; Saito, Takumi*
Applied Geochemistry, 152, p.105620_1 - 105620_11, 2023/05
Times Cited Count:1 Percentile:56.68(Geochemistry & Geophysics)Rizaal, M.; Nakajima, Kunihisa; Saito, Takumi*; Osaka, Masahiko; Okamoto, Koji*
ACS Omega (Internet), 7(33), p.29326 - 29336, 2022/08
Times Cited Count:3 Percentile:27.64(Chemistry, Multidisciplinary)Tachi, Yukio; Saito, Takumi*; Kirishima, Akira*
Nihon Genshiryoku Gakkai-Shi ATOMO , 64(5), p.290 - 295, 2022/05
, 64(5), p.290 - 295, 2022/05
no abstracts in English
 C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticles
C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticlesFueda, Kazuki*; Takami, Ryu*; Minomo, Kenta*; Morooka, Kazuya*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Saito, Takumi*; Shiotsu, Hiroyuki; Onuki, Toshihiko*; et al.
Journal of Hazardous Materials, 428, p.128214_1 - 128214_10, 2022/04
Times Cited Count:7 Percentile:66.01(Engineering, Environmental)Mei, H.; Aoyagi, Noboru; Saito, Takumi*; Kozai, Naofumi; Sugiura, Yuki; Tachi, Yukio
Applied Geochemistry, 136, p.105178_1 - 105178_8, 2022/01
Times Cited Count:11 Percentile:90.47(Geochemistry & Geophysics) and Tb
and Tb measured by gel electrophoresis techniques
measured by gel electrophoresis techniquesNakano, Sumika*; Marumo, Kazuki*; Kazami, Rintaro*; Saito, Takumi*; Haraga, Tomoko; Tasaki-Handa, Yuiko*; Saito, Shingo*
Environmental Science & Technology, 55(22), p.15172 - 15180, 2021/11
Times Cited Count:5 Percentile:33.8(Engineering, Environmental)Humic acid (HA) can strongly complex with metal ions to form a supramolecular assembly via coordination binding. However, determining the supramolecular size distribution and stoichiometry between small HA unit molecules constituting HA supramolecule and metal ions has proven to be challenging. Here, we investigated the changes in the size distributions of HAs induced by Cu and Tb
and Tb ions using a unique polyacrylamide gel electrophoresis (PAGE) for the separation and quantification of HA complexes and metal ions bound, followed by UV-Vis spectroscopy and EEM-PARAFAC. It was found that the supramolecular behaviors of Cu
ions using a unique polyacrylamide gel electrophoresis (PAGE) for the separation and quantification of HA complexes and metal ions bound, followed by UV-Vis spectroscopy and EEM-PARAFAC. It was found that the supramolecular behaviors of Cu and Tb
and Tb complexes with HA collected from peat and deep groundwater (HHA) differed. Our results suggest that this supramolecular stoichiometry is related to the abundance of sulfur atoms in the elemental composition of HHA. Our results provide new insights into HA supramolecules formed via metal complexation.
complexes with HA collected from peat and deep groundwater (HHA) differed. Our results suggest that this supramolecular stoichiometry is related to the abundance of sulfur atoms in the elemental composition of HHA. Our results provide new insights into HA supramolecules formed via metal complexation.
Zhou, Q.*; Saito, Takumi*; Suzuki, Seiya; Yano, Kimihiko; Suzuki, Shunichi*
Journal of Nuclear Science and Technology, 58(4), p.461 - 472, 2021/04
Times Cited Count:6 Percentile:70.8(Nuclear Science & Technology)Saeki, Morihisa*; Yomogida, Takumi; Matsumura, Daiju; Saito, Takumi*; Nakanishi, Ryuzo*; Tsuji, Takuya; Oba, Hironori*
Analytical Sciences, 36(11), p.1371 - 1378, 2020/11
Times Cited Count:2 Percentile:9.69(Chemistry, Analytical)We measured X-ray absorption fine structure (XAFS) and Raman spectra of isopolymolybdates(VI) in HNO solution (0.15- 4.0 M), which change their geometries depending on acid concentration, and performed simultaneous resolution of the XAFS and Raman data using a multivariate curve resolution by alternating least-squares (MCR-ALS) analysis. In iterative ALS optimization, initial data matrices were prepared by two different methods. The MCR-ALS result of single XAFS data matrix shows large dependence on the preparation method of the initial data matrices. The MCR-ALS result of an augmented matrix of Raman and XAFS data has little dependence on the initial data matrices. It indicates that the augmentation method effectively improves the rotation ambiguities in the MCR-ALS analysis of the XAFS data. Based on the model fitting of the pure EXAFS oscillations, we revealed the change of [Mo
solution (0.15- 4.0 M), which change their geometries depending on acid concentration, and performed simultaneous resolution of the XAFS and Raman data using a multivariate curve resolution by alternating least-squares (MCR-ALS) analysis. In iterative ALS optimization, initial data matrices were prepared by two different methods. The MCR-ALS result of single XAFS data matrix shows large dependence on the preparation method of the initial data matrices. The MCR-ALS result of an augmented matrix of Raman and XAFS data has little dependence on the initial data matrices. It indicates that the augmentation method effectively improves the rotation ambiguities in the MCR-ALS analysis of the XAFS data. Based on the model fitting of the pure EXAFS oscillations, we revealed the change of [Mo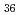 O
O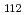 (H
(H O)
O)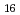 ]
]
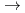 [Mo
[Mo O
O (H
(H O)
O) ]
]
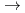 [HMoO
[HMoO (H
(H O)
O) ]
] in the highly concentrated HNO
in the highly concentrated HNO solution.
solution.
Rizaal, M.; Nakajima, Kunihisa; Saito, Takumi*; Osaka, Masahiko; Okamoto, Koji*
Journal of Nuclear Science and Technology, 57(9), p.1062 - 1073, 2020/09
Times Cited Count:8 Percentile:70.65(Nuclear Science & Technology)The interaction of cesium hydroxide and a calcium silicate insulation material was experimentally investigated at high temperature conditions. A thermogravimetry equipped with differential thermal analysis was used to analyze thermal events in the samples of mixed calcium silicate and cesium hydroxide under Ar-5%H and Ar-4%H
and Ar-4%H -20%H
-20%H 0 with maximum temperature of 1100
0 with maximum temperature of 1100 C. Prior being mixed with cesium hydroxide, a part of calcium silicate was pretreated at high temperature to evaluate the effect of possible structural changes of this material due to a preceding thermal history and also the sake of thermodynamic evaluation to those available ones. Based upon the initial condition (preliminary heat treatment) of calcium silicate, it was found that if the original material consisted of xonotlite (Ca
C. Prior being mixed with cesium hydroxide, a part of calcium silicate was pretreated at high temperature to evaluate the effect of possible structural changes of this material due to a preceding thermal history and also the sake of thermodynamic evaluation to those available ones. Based upon the initial condition (preliminary heat treatment) of calcium silicate, it was found that if the original material consisted of xonotlite (Ca Si
Si 0
0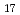 (0H)
(0H) ), the endothermic reaction with cesium hydroxide occurred over the temperature range 575-730
), the endothermic reaction with cesium hydroxide occurred over the temperature range 575-730 C meanwhile if the crystal phase of original material was changed to wollastonite (CaSi0
C meanwhile if the crystal phase of original material was changed to wollastonite (CaSi0 ), the interaction occurred over temperature range 700-1100
), the interaction occurred over temperature range 700-1100 C. Furthermore, the X-ray diffraction analyses have indicated on both type of pretreated calsils that regardless of Ar-5%H
C. Furthermore, the X-ray diffraction analyses have indicated on both type of pretreated calsils that regardless of Ar-5%H and Ar-4%H
and Ar-4%H -20%H
-20%H 0 atmosphere, cesium aluminum silicate, CsAlSi0
0 atmosphere, cesium aluminum silicate, CsAlSi0 was formed with aluminum in the samples as an impurity or adduct.
was formed with aluminum in the samples as an impurity or adduct.
Rizaal, M.; Saito, Takumi*; Okamoto, Koji*; Erkan, N.*; Nakajima, Kunihisa; Osaka, Masahiko
Mechanical Engineering Journal (Internet), 7(3), p.19-00563_1 - 19-00563_10, 2020/06
The adsorption of cesium (Cs) on calcium silicate insulation of primary piping system is postulated to contribute in high dose rate of surrounding pedestal area in Fukushima Daiichi NPP unit 2. In this study, room-temperature experiment of Cs adsorption on calcium silicate has been studied as an initial approach of Cs adsorption behavior toward higher temperature condition. As the result of analyzing of Cs adsorption kinetics, it was expected that the underlying adsorption mechanism is chemisorption. Furthermore, analysis of adsorption isotherm suggested unrestricted monolayer formation followed by multilayer formation.
 -humate complexes
-humate complexesMarumo, Kazuki*; Matsumoto, Atsumasa*; Nakano, Sumika*; Shibukawa, Masami*; Saito, Takumi*; Haraga, Tomoko; Saito, Shingo*
Environmental Science & Technology, 53(24), p.14507 - 14515, 2019/12
Times Cited Count:7 Percentile:28.45(Engineering, Environmental)Humic acids (HA) are responsible for the fate of metal ions in the environment. We developed a polyacrylamide gel electrophoresis (PAGE) technique to investigate the MW distributions of metal ion (copper ion). Combining contaminant-metal-free and high-resolution PAGE systems for HA provided accurate MW distributions for the metal ions. Coupling this system with UV-Vis spectrometry and the excitation-emission matrix (EEM) spectrometry-parallel factor analysis (PARAFAC) method revealed new insights into metal-HA complex. Interestingly, the MW distributions of the three metal ions were entirely different, indicating that the presence of specific binding environments in HA for the metal ions depending its MW. The MW distributions of five fluorescent components were associated with the metal ion distributions. Our PAGE-based methodology suggests that metal binding sites and fluorescent components in HA exhibit heterogeneity in terms of metal binding affinity and MW.
 , Zn
, Zn +, and Co
+, and Co
Kato, Tomoaki*; Yu, Q.*; Tanaka, Kazuya; Kozai, Naofumi; Saito, Takumi*; Onuki, Toshihiko
Journal of Environmental Sciences, 86, p.78 - 86, 2019/12
Times Cited Count:2 Percentile:8.23(Environmental Sciences)This paper investigated the fate of the dissolved permanganate in aqueous solution after contact with bacterial cells and metal accumulation during precipitation of Mn oxides. When Mn(VII) was contacted with bacterial cells, cells were damaged and Mn(VII) was reduced by cells to lower valence and precipitated as Mn oxides (biomass Mn oxides). When Co ions were present, Co was incorporated into Mn oxides as Co
ions were present, Co was incorporated into Mn oxides as Co . These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
. These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
Kobayashi, Taishi*; Nakajima, Shogo*; Motokawa, Ryuhei; Matsumura, Daiju; Saito, Takumi*; Sasaki, Takayuki*
Langmuir, 35(24), p.7995 - 8006, 2019/06
Times Cited Count:5 Percentile:22.07(Chemistry, Multidisciplinary)Saito, Takumi*; Aoyagi, Noboru; Terashima, Motoki
Journal of Nuclear Science and Technology, 54(4), p.444 - 451, 2017/04
Times Cited Count:6 Percentile:50.9(Nuclear Science & Technology)Humic substances (HSs) are ubiquitous in various environments including deep underground and play an important role in the speciation and mobility of radionuclides. The binding of Eu , a chemical homologue of trivalent actinide ions, to HSs isolated from sedimentary groundwater at -250 m below the surface was studied by time-resolved laser fluorescence spectroscopy combined with parallel factor analysis (PARAFAC) as a function of pH and salt concentration. PARAFAC modeling reveals the presence of multiple factors that corresponds to different Eu
, a chemical homologue of trivalent actinide ions, to HSs isolated from sedimentary groundwater at -250 m below the surface was studied by time-resolved laser fluorescence spectroscopy combined with parallel factor analysis (PARAFAC) as a function of pH and salt concentration. PARAFAC modeling reveals the presence of multiple factors that corresponds to different Eu species. These factors resemble those observed for Eu
species. These factors resemble those observed for Eu binding to HSs from surface environments; however, detailed comparison shows that there are some particularities in Eu
binding to HSs from surface environments; however, detailed comparison shows that there are some particularities in Eu binding to the deep groundwater HSs. The distribution coefficients (
binding to the deep groundwater HSs. The distribution coefficients (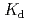 ) of Eu
) of Eu binding to the HSs calculated from the PARAFAC modeling exhibits a rather strong salt effect. At 0.01 M NaClO
binding to the HSs calculated from the PARAFAC modeling exhibits a rather strong salt effect. At 0.01 M NaClO the
the 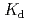 values are relatively large and comparable to those to the surface HSs; they are decreaed at 0.1 M NaClO
values are relatively large and comparable to those to the surface HSs; they are decreaed at 0.1 M NaClO by more than an order of the magnitude. The
by more than an order of the magnitude. The 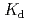 values are larger for humic acid fraction of the deep underground HSs than fulvic acid over the entire range of pH and salt concentration investigated in this study.
values are larger for humic acid fraction of the deep underground HSs than fulvic acid over the entire range of pH and salt concentration investigated in this study.
 on Na-montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modeling
on Na-montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modelingSasaki, Takayuki*; Ueda, Kenyo*; Saito, Takumi; Aoyagi, Noboru; Kobayashi, Taishi*; Takagi, Ikuji*; Kimura, Takaumi; Tachi, Yukio
Journal of Nuclear Science and Technology, 53(4), p.592 - 601, 2016/04
Times Cited Count:12 Percentile:74.37(Nuclear Science & Technology)The influences of pH and the concentrations of Eu and NaNO
and NaNO on the sorption of Eu
on the sorption of Eu to Na-montmorillonite were investigated through batch sorption measurements and time-resolved laser fluorescence spectroscopy (TRLFS). The pH had a little effect on the distribution coefficients (Kd) in 0.01 M NaNO
to Na-montmorillonite were investigated through batch sorption measurements and time-resolved laser fluorescence spectroscopy (TRLFS). The pH had a little effect on the distribution coefficients (Kd) in 0.01 M NaNO , whereas the Kd strongly depended on pH at 1 M NaNO
, whereas the Kd strongly depended on pH at 1 M NaNO . A cation exchange model combined with a one-site non-electrostatic surface complexation model was successfully applied to the measured Kd. The TRLFS spectra of Eu
. A cation exchange model combined with a one-site non-electrostatic surface complexation model was successfully applied to the measured Kd. The TRLFS spectra of Eu sorbed were processed by parallel factor analysis (PARAFAC), which corresponded to one outer-sphere (factor A) and two inner-sphere (factor B and C) complexes. It turned out that factors A and B correspond to Eu
sorbed were processed by parallel factor analysis (PARAFAC), which corresponded to one outer-sphere (factor A) and two inner-sphere (factor B and C) complexes. It turned out that factors A and B correspond to Eu sorbed by ion exchange sites and inner-sphere complexation with hydroxyl groups of the edge faces, respectively. Factor C became dominant at relatively high pH and ionic strength and likely correspond to the precipitation of Eu(OH)
sorbed by ion exchange sites and inner-sphere complexation with hydroxyl groups of the edge faces, respectively. Factor C became dominant at relatively high pH and ionic strength and likely correspond to the precipitation of Eu(OH) on the surface.
on the surface.
Nagasaki, Shinya*; Saito, Takumi; Yang, T.*
Journal of Radioanalytical and Nuclear Chemistry, 308(1), p.143 - 153, 2016/04
Times Cited Count:13 Percentile:76.89(Chemistry, Analytical)Sorption of Np(V) on illite, shale and MX-80 under oxidizing conditions were first studied in two types of high ionic strength solutions: (1) a reference brine solution (SR-270-PW) with an ionic strength (I) of 6.0 M, and (2) Na-Ca-Cl solutions (with different Na/Ca molar ratios and different ionic strengths, up to a maximum of 4.6 M). The effects of pHc, Na/Ca ratio, and ionic strength on sorption in Na-Ca-Cl solutions were investigated. The Kd values increased with increasing pHc and Na/Ca ratio for all sorbents studied. The Kd values on illite and shale were independent of the ionic strength over the range 0.10-4.6 M, and the Kd value on MX-80 was independent of ionic strength greater than 1.0 M. The Kd values and the sorption isotherms in both SR-270-PW and Na-Ca-Cl solutions were also studied.
Yamashita, Yuji*; Saito, Takumi
Journal of Environmental Chemical Engineering, 3(4), p.3024 - 3029, 2015/12
Humic substances are natural organic matters with heterogeneity in the size distribution and composition of there functional groups. In this study, the effects of organic acid, which are often used as pH buffering agents, on the size of a humic substance and size-dependent binding of metal ions on the humic substance were investigated by flow-field flow fractionation (Fl-FFF). Comparing the three organic acids, Tris, MES, and MOPS, the size of purified Aldrich humic acid (PAHA) changed in the presence of Tris and MES, suggesting that the interaction with these organic acid modulated the molecular structure of PAHA; on the other hand, MOPS hardly affect the size of PAHA. Size-dependent binding of europium (Eu ) and uranium (UO
) and uranium (UO
 ) were also studied by Fl-FFF in the presence of MOPS. The binding of these metal ions were not homogeneous with respect to the size of PAHA; they exhibited high affinity to PAHA fractions with 5 nm hydrodynamic diameter.
) were also studied by Fl-FFF in the presence of MOPS. The binding of these metal ions were not homogeneous with respect to the size of PAHA; they exhibited high affinity to PAHA fractions with 5 nm hydrodynamic diameter.