Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -glucanase from
-glucanase from 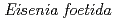
Arimori, Takao*; Ito, Akihiro*; Nakazawa, Masami*; Ueda, Mitsuhiro*; Tamada, Taro
Journal of Synchrotron Radiation, 20(6), p.884 - 889, 2013/11
Times Cited Count:17 Percentile:65.54(Instruments & Instrumentation)The saccharification process is essential for bioethanol production from woody biomass including celluloses. Cold-adapted cellulase, which has sufficient activity at low temperature ( 293 K), is capable of reducing heating costs during the saccharification process and is suitable for simultaneous saccharification and fermentation. Endo-1,4-
293 K), is capable of reducing heating costs during the saccharification process and is suitable for simultaneous saccharification and fermentation. Endo-1,4- -glucanase from the earthworm Eisenia fetida (EF-EG2) belonging to glycoside hydrolase family 9 has been shown to have the highest activity at 313 K, and also retained a comparatively high activity at 283 K. The recombinant EF-EG2 was purified expressed in Pichia pastoris, and then grew needle-shaped crystals with dimensions of 0.02
-glucanase from the earthworm Eisenia fetida (EF-EG2) belonging to glycoside hydrolase family 9 has been shown to have the highest activity at 313 K, and also retained a comparatively high activity at 283 K. The recombinant EF-EG2 was purified expressed in Pichia pastoris, and then grew needle-shaped crystals with dimensions of 0.02  0.02
0.02  1 mm. The crystals belonged to the space group P3221 with unit-cell parameters of
1 mm. The crystals belonged to the space group P3221 with unit-cell parameters of 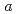 =
= 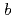 =136
=136  ,
, 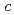 = 55.0
= 55.0  ,. The final model of EF-EG2, including 435 residues, two ions, seven crystallization reagents and 696 waters, was refined to a crystallographic
,. The final model of EF-EG2, including 435 residues, two ions, seven crystallization reagents and 696 waters, was refined to a crystallographic 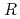 -factor of 14.7% (free
-factor of 14.7% (free 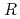 -factor of 16.8%) to 1.5
-factor of 16.8%) to 1.5  , resolution. The overall structure of EF-EG2 has an (
, resolution. The overall structure of EF-EG2 has an ( /
/ )
) barrel fold which contains a putative active-site cleft and a negatively charged surface. This structural information helps us understand the catalytic and cold adaptation mechanisms of EF-EG2.
barrel fold which contains a putative active-site cleft and a negatively charged surface. This structural information helps us understand the catalytic and cold adaptation mechanisms of EF-EG2.
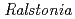 sp. A-471 reveals a unique arrangement of the catalytic residues for inverting chitin hydrolysis
sp. A-471 reveals a unique arrangement of the catalytic residues for inverting chitin hydrolysisArimori, Takao*; Kawamoto, Noriko*; Shinya, Shoko*; Okazaki, Nobuo*; Nakazawa, Masami*; Miyatake, Kazutaka*; Fukamizo, Tamo*; Ueda, Mitsuhiro*; Tamada, Taro
Journal of Biological Chemistry, 288(26), p.18696 - 18706, 2013/07
Times Cited Count:30 Percentile:64.37(Biochemistry & Molecular Biology)Chitinase C from 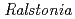 sp. A-471 (Ra-ChiC) has a catalytic domain sequence similar to goose type (G-type) lysozymes and, unlike other chitinases, belongs to glycohydrolase (GH) family 23. Using NMR spectroscopy, however, Ra-ChiC was found to interact only with the chitin dimer but not with the peptideglycan fragment. Here we report the crystal structures of wild-type, E141Q, and E162Q of the catalytic domain of Ra-ChiC with or without chitin oligosaccharides. Ra-ChiC has a substrate-binding site including a tunnel-shaped cavity, which determines the substrate specificity. Mutation analyses based on this structural information indicated that a highly conserved Glu141 acts as a catalytic acid, and that Asp226 located at the roof of the tunnel activates a water molecule as a catalytic base. The unique arrangement of the catalytic residues makes a clear contrast to the other GH23 members and also to inverting GH19 chitinases.
sp. A-471 (Ra-ChiC) has a catalytic domain sequence similar to goose type (G-type) lysozymes and, unlike other chitinases, belongs to glycohydrolase (GH) family 23. Using NMR spectroscopy, however, Ra-ChiC was found to interact only with the chitin dimer but not with the peptideglycan fragment. Here we report the crystal structures of wild-type, E141Q, and E162Q of the catalytic domain of Ra-ChiC with or without chitin oligosaccharides. Ra-ChiC has a substrate-binding site including a tunnel-shaped cavity, which determines the substrate specificity. Mutation analyses based on this structural information indicated that a highly conserved Glu141 acts as a catalytic acid, and that Asp226 located at the roof of the tunnel activates a water molecule as a catalytic base. The unique arrangement of the catalytic residues makes a clear contrast to the other GH23 members and also to inverting GH19 chitinases.
Arimori, Takao; Yamagata, Yuriko*
Fukuoka Igaku Zasshi, 102(11), p.303 - 312, 2011/11
Human NUDT5 hydrolyzes 8-oxo-dGDP into 8-oxo-dGMP and inorganic phosphate and prevents mutations caused by misincorporation of 8-oxoG into DNA. In addition, NUDT5 displays a broad substrate specificity for various modified nucleotides including 8-oxo-dADP, 8-oxo-GDP, and ADP-ribose. To understand the mechanisms of substrate recognition and catalysis by NUDT5, we have determined the crystal structure of NUDT5 complexed with 8-oxo-dGDP. A comparison of the structure with the previously reported NUDT5-ADP-ribose complex structure illustrated extremely different binding modes of these substrates. Especially, unexpectedly, the positions of two phosphates of substrate ( and
and  phosphates) in the 8-oxo-dGDP complex are completely inverted compared to those in the ADP-ribose complex, which indicates that either the catalytic residues or the nucleophilic substitution sites of substrates are different between hydrolysis reactions of these substrates. To further investigate the catalytic mechanisms, we next determined the site of nucleophilic substitution of water for 8-oxo-dGDP and ADP-ribose by the
phosphates) in the 8-oxo-dGDP complex are completely inverted compared to those in the ADP-ribose complex, which indicates that either the catalytic residues or the nucleophilic substitution sites of substrates are different between hydrolysis reactions of these substrates. To further investigate the catalytic mechanisms, we next determined the site of nucleophilic substitution of water for 8-oxo-dGDP and ADP-ribose by the 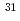 P NMR spectra of the reaction products in
P NMR spectra of the reaction products in 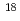 O-labeled water. The results clearly showed that the sites were different between these substrates, that is, 8-oxo-dGDP was attacked by a nucleophilic water at the P
O-labeled water. The results clearly showed that the sites were different between these substrates, that is, 8-oxo-dGDP was attacked by a nucleophilic water at the P , whereas ADP-ribose was at the P
, whereas ADP-ribose was at the P . Thus, we revealed the unique catalytic mechanism of NUDT5 as well as the multiple substrate recognition mechanism by structural and NMR analyses.
. Thus, we revealed the unique catalytic mechanism of NUDT5 as well as the multiple substrate recognition mechanism by structural and NMR analyses.
Arimori, Takao; Tamaoki, Haruhiko*; Nakamura, Teruya*; Kamiya, Hiroyuki*; Ikemizu, Shinji*; Takagi, Yasumitsu*; Ishibashi, Toru*; Harashima, Hideyoshi*; Sekiguchi, Mutsuo*; Yamagata, Yuriko*
Nucleic Acids Research, 39(20), p.8972 - 8983, 2011/11
Times Cited Count:24 Percentile:52.33(Biochemistry & Molecular Biology)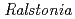 sp. A-471
sp. A-471Okazaki, Nobuo; Arimori, Takao; Nakazawa, Masami*; Miyatake, Kazutaka*; Ueda, Mitsuhiro*; Tamada, Taro
Acta Crystallographica Section F, 67(4), p.494 - 497, 2011/04
Times Cited Count:3 Percentile:41.34(Biochemical Research Methods)Arimori, Takao; Okazaki, Nobuo; Nakazawa, Masami*; Miyatake, Kazutaka*; Ueda, Mitsuhiro*; Tamada, Taro
no journal, ,
no abstracts in English
Arimori, Takao; Tamaoki, Haruhiko*; Nakamura, Teruya*; Kamiya, Hiroyuki*; Ikemizu, Shinji*; Takagi, Yasumitsu*; Ishibashi, Toru*; Harashima, Hideyoshi*; Sekiguchi, Mutsuo*; Yamagata, Yuriko*
no journal, ,
no abstracts in English
Arimori, Takao; Kawamoto, Noriko*; Okazaki, Nobuo; Nakazawa, Masami*; Miyatake, Kazutaka*; Ueda, Mitsuhiro*; Tamada, Taro
no journal, ,
no abstracts in English
 -glucanase from Eisenia foetida
-glucanase from Eisenia foetidaArimori, Takao*; Ito, Akihiro*; Nakazawa, Masami*; Ueda, Mitsuhiro*; Tamada, Taro
no journal, ,
 -endoglucanase from
-endoglucanase from 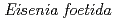
Arimori, Takao*; Ito, Akihiro*; Nakazawa, Masami*; Ueda, Mitsuhiro*; Tamada, Taro
no journal, ,
no abstracts in English
Arimori, Takao*; Kawamoto, Noriko*; Okazaki, Nobuo*; Nakazawa, Masami*; Miyatake, Kazutaka*; Fukamizo, Tamo*; Ueda, Mitsuhiro*; Tamada, Taro
no journal, ,
Chitin, linear  -1,4-linked polymer of
-1,4-linked polymer of  -acetyl-D-glucosamine (NAG), is the second abundant biopolymer in nature next to cellulose. Hydrolysis of chitin provides useful products,
-acetyl-D-glucosamine (NAG), is the second abundant biopolymer in nature next to cellulose. Hydrolysis of chitin provides useful products,  -acetyl-chitooligosaccharides [(NAG)
-acetyl-chitooligosaccharides [(NAG)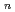 ] and chitooligosaccharides, which have a variety of biological functions including antibacterial activity and antitumor activity. We have previously cloned a novel chitinase gene from a moderate thermophilic strain
] and chitooligosaccharides, which have a variety of biological functions including antibacterial activity and antitumor activity. We have previously cloned a novel chitinase gene from a moderate thermophilic strain 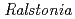 sp. A-471 (Ra-ChiC). Ra-ChiC comprises a signal peptide, a chitin-binding domain, an interdomain linker, and a catalytic domain. The catalytic domain shares amino acid sequence homology with goose type (G-type) lysozymes and, unlike other chitinases, Ra-ChiC belongs to glycohydrolase (GH) family 23. However, Ra-ChiC does not exhibit lysozyme activity, but only chitinase activity. In this study, we aim to reveal how Ra-ChiC catalyzes the hydrolysis of chitin and why Ra-ChiC exhibits chitinase activity instead of lysozyme activity. We determined the crystal structures of the catalytic domain of Ra-ChiC (Ra-ChiC
sp. A-471 (Ra-ChiC). Ra-ChiC comprises a signal peptide, a chitin-binding domain, an interdomain linker, and a catalytic domain. The catalytic domain shares amino acid sequence homology with goose type (G-type) lysozymes and, unlike other chitinases, Ra-ChiC belongs to glycohydrolase (GH) family 23. However, Ra-ChiC does not exhibit lysozyme activity, but only chitinase activity. In this study, we aim to reveal how Ra-ChiC catalyzes the hydrolysis of chitin and why Ra-ChiC exhibits chitinase activity instead of lysozyme activity. We determined the crystal structures of the catalytic domain of Ra-ChiC (Ra-ChiC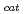 ), Ra-ChiC
), Ra-ChiC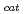 complexed with (NAG)
complexed with (NAG) , E141Q mutant of Ra-ChiC
, E141Q mutant of Ra-ChiC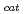 complexed with (NAG)
complexed with (NAG) , E162Q mutant of Ra-ChiC
, E162Q mutant of Ra-ChiC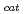 , and E162Q mutant of Ra-ChiC
, and E162Q mutant of Ra-ChiC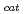 complexed with (NAG)
complexed with (NAG) . These structures provided us structural basis of substrate recognition mechanism and revealed that Ra-ChiC has a unique substrate-binding site including a tunnel-shaped cavity, which determines the substrate specificity. In addition, we also carried out a mutation analysis of acidic amino acid residues located at the active site. As a result, we found that not only a highly conserved Glu141 but also Asp226 located at the roof of the tunnel have quite important roles in catalysis.
. These structures provided us structural basis of substrate recognition mechanism and revealed that Ra-ChiC has a unique substrate-binding site including a tunnel-shaped cavity, which determines the substrate specificity. In addition, we also carried out a mutation analysis of acidic amino acid residues located at the active site. As a result, we found that not only a highly conserved Glu141 but also Asp226 located at the roof of the tunnel have quite important roles in catalysis.
Tamada, Taro; Arimori, Takao*; Fukuhara, Hiroaki*; Ito, Akihiro*; Ueda, Mitsuhiro*
no journal, ,
The saccharification process is essential for bioethanol production from woody biomass including celluloses. Cold-adapted cellulase, which has sufficient activity at low temperature ( 293 K), is capable of reducing heating costs during the saccharification process and is suitable for simultaneous saccharification and fermentation. Endo-1,4-
293 K), is capable of reducing heating costs during the saccharification process and is suitable for simultaneous saccharification and fermentation. Endo-1,4- -glucanase from the earthworm Eisenia fetida (EF-EG2) belonging to glycoside hydrolase family 9 has been shown to have the highest activity at 313 K, and also retained a comparatively high activity at 283 K. The recombinant EF-EG2, expressed in
-glucanase from the earthworm Eisenia fetida (EF-EG2) belonging to glycoside hydrolase family 9 has been shown to have the highest activity at 313 K, and also retained a comparatively high activity at 283 K. The recombinant EF-EG2, expressed in 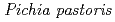 , was purified and then grew needle-shaped crystals with dimensions of 0.02
, was purified and then grew needle-shaped crystals with dimensions of 0.02  0.02
0.02  1 mm. The final model of EF-EG2, including 435 residues, two ions, seven crystallization reagents and 696 waters, was refined to a crystallographic
1 mm. The final model of EF-EG2, including 435 residues, two ions, seven crystallization reagents and 696 waters, was refined to a crystallographic 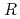 -factor of 14.7% (free
-factor of 14.7% (free 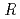 -factor of 16.8%) to 1.5
-factor of 16.8%) to 1.5  resolution. The overall structure of EF-EG2 has an(
resolution. The overall structure of EF-EG2 has an( /
/ )
) barrel fold which contains a putative active-site cleft and a negatively charged surface. Furthermore, in the structure of the E431Q mutant of EF-EG2 complexed with cellotriose (CTR), electron densities corresponding to CTR are observed well at -4 to -2 subsites. This structural information helps us understand the catalytic and cold adaptation mechanisms of EF-EG2.
barrel fold which contains a putative active-site cleft and a negatively charged surface. Furthermore, in the structure of the E431Q mutant of EF-EG2 complexed with cellotriose (CTR), electron densities corresponding to CTR are observed well at -4 to -2 subsites. This structural information helps us understand the catalytic and cold adaptation mechanisms of EF-EG2.