Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Cantarel, V.; Arisaka, Makoto; Yamagishi, Isao
Journal of the American Ceramic Society, 102(12), p.7553 - 7563, 2019/12
Times Cited Count:8 Percentile:29.81(Materials Science, Ceramics)The hydrogen gas (H ) production of wasteforms is a major safety concern for encapsulating nuclear wastes. For geopolymers, the H
) production of wasteforms is a major safety concern for encapsulating nuclear wastes. For geopolymers, the H produced by radiolytic processes is a key factor because of the large amount of water present in their porous structure. Herein, the hydrogen production was measured under
produced by radiolytic processes is a key factor because of the large amount of water present in their porous structure. Herein, the hydrogen production was measured under  Co gamma irradiation. The effect of water saturation and sample size were studied for pure geopolymers, or using zeolites as an example waste. When geopolymer monolithic samples were large and saturated by water, the hydrogen released was measured up to two orders of magnitude lower with a 40 cm long cylinder samples (1.9
Co gamma irradiation. The effect of water saturation and sample size were studied for pure geopolymers, or using zeolites as an example waste. When geopolymer monolithic samples were large and saturated by water, the hydrogen released was measured up to two orders of magnitude lower with a 40 cm long cylinder samples (1.9 10
10 mol/J) than a sample in powder form (2.2
mol/J) than a sample in powder form (2.2 10
10 mol/J). To interpret results, a simple model was used, considering only hydrogen production, a potential recombination and its diffusion in the geopolymer matrix. Knowing the diffusion constant of the matrix, the model was able to reproduce the evolution of the hydrogen release as a function of the water saturation level and predict the evolution when sample size is increased up to 40 cm.
mol/J). To interpret results, a simple model was used, considering only hydrogen production, a potential recombination and its diffusion in the geopolymer matrix. Knowing the diffusion constant of the matrix, the model was able to reproduce the evolution of the hydrogen release as a function of the water saturation level and predict the evolution when sample size is increased up to 40 cm.
Arisaka, Makoto
JAEA-Research 2018-014, 27 Pages, 2019/02
Influence of ultrasonic irradiation on cesium (Cs) retention ability of biotite was examined in order to support of management of wastes generated by the Fukushima Daiichi Nuclear Power Station accident. Suspensions of Cs exchanged biotite were ultrasonically irradiated at three frequencies of 200, 430, and 950 kHz. The concentration of Cs in the aqueous phase increased, when the irradiation frequency of the ultrasonic is 430 kHz, compared with that without irradiation. This result means decrease of Cs retention ability of biotite. In addition, we observed two phenomena, that (i) the stability of suspension decreased after ultrasonic irradiation and (ii) the Cs concentration continued to increase after the irradiation. However, phenomena were hardly reproducible with a limited of experiments.
Cantarel, V.; Arisaka, Makoto; Yamagishi, Isao
Proceedings of 3rd International Symposium on Cement-based Materials for Nuclear Wastes (NUWCEM 2018) (USB Flash Drive), 4 Pages, 2018/10
Geopolymers were successfully synthesized with different water content and loading of zeolites (0 to 40%wt). Zeolite A, geopolymer and their composites were then irradiated by a  Co source (1.5 to 2.5 kGy/h) up to 10 kGy. The hydrogen was measured by GC after irradiation. Yields of radiolitic hydrogen were obtained for individual components. Obtained yields suggested a recombination process during irradiation. The system was then modelled to explain the observed behavior and predict the hydrogen production under
Co source (1.5 to 2.5 kGy/h) up to 10 kGy. The hydrogen was measured by GC after irradiation. Yields of radiolitic hydrogen were obtained for individual components. Obtained yields suggested a recombination process during irradiation. The system was then modelled to explain the observed behavior and predict the hydrogen production under  ray irradiation of larger samples.
ray irradiation of larger samples.
 -rays
-raysArisaka, Makoto; Watanabe, Masayuki; Ishizaki, Manabu*; Kurihara, Masato*; Chen, R.*; Tanaka, Hisashi*
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1543 - 1547, 2015/02
Times Cited Count:13 Percentile:70.51(Chemistry, Analytical)The influence of irradiation with  -rays to metal hexacyanoferrate (MHCF: M = Fe, Cu or Ni), which is known as an adsorbent for selective adsorption of cesium (Cs) ion in solution, on Cs adsorption ability and stability was investigated in HNO
-rays to metal hexacyanoferrate (MHCF: M = Fe, Cu or Ni), which is known as an adsorbent for selective adsorption of cesium (Cs) ion in solution, on Cs adsorption ability and stability was investigated in HNO solutions. Under the adsorbed dose conditions (50-300 kGy), it was found that the MHCF is fully stable although the radiolytic decomposition of MHCF was slightly observed with an increase of the total adsorbed dose, which was confirmed by an increment of Fe, Cu or Ni concentration in HNO
solutions. Under the adsorbed dose conditions (50-300 kGy), it was found that the MHCF is fully stable although the radiolytic decomposition of MHCF was slightly observed with an increase of the total adsorbed dose, which was confirmed by an increment of Fe, Cu or Ni concentration in HNO solution after the irradiation. The weight percent of the metal in the solution to initial weight of MHCF was less than unity. Moreover, no change in composition of carbon, hydrogen and nitrogen in MHCF was observed. On the other hand, the distribution coefficients of Cs to the irradiated MHCF were independent of the total adsorbed dose. This indicates that the Cs adsorption ability was maintained under
solution after the irradiation. The weight percent of the metal in the solution to initial weight of MHCF was less than unity. Moreover, no change in composition of carbon, hydrogen and nitrogen in MHCF was observed. On the other hand, the distribution coefficients of Cs to the irradiated MHCF were independent of the total adsorbed dose. This indicates that the Cs adsorption ability was maintained under  -ray irradiation.
-ray irradiation.
 -ray irradiation
-ray irradiationArisaka, Makoto; Watanabe, Masayuki; Sugo, Yumi; Kobayashi, Kumiko*; Kanao, Osamu*; Kimura, Takaumi
Journal of Nuclear Science and Technology, 51(4), p.457 - 464, 2014/04
Times Cited Count:3 Percentile:23.92(Nuclear Science & Technology)Toward the development of a practical separation method for trivalent actinides and lanthanides, we used extraction chromatography with alkyl-pyridinedicarboxyamides as the extractant. The results confirmed that the performance degradation of the adsorbent caused by contact with HNO and/or irradiation with
and/or irradiation with  rays would be very small during the operation of column chromatography. The optimal conditions for the column separation were also determined: eluent, 5M HNO
rays would be very small during the operation of column chromatography. The optimal conditions for the column separation were also determined: eluent, 5M HNO ; flow rate, 0.1 mL/min.
; flow rate, 0.1 mL/min.
 adsorption
adsorption  lattice defect sites of Prussian blue filled with coordination and crystallization water molecules
lattice defect sites of Prussian blue filled with coordination and crystallization water moleculesIshizaki, Manabu*; Akiba, Sae*; Otani, Asako*; Hoshi, Yuji*; Ono, Kenta*; Matsuba, Mayu*; Togashi, Takanari*; Kanaizuka, Katsuhiko*; Sakamoto, Masatomi*; Takahashi, Akira*; et al.
Dalton Transactions, 42(45), p.16049 - 16055, 2013/12
Times Cited Count:178 Percentile:99.58(Chemistry, Inorganic & Nuclear)We have revealed the fundamental mechanism of specific Cs adsorption into Prussian blue (PB) in order to develop high-performance PB-based Cs
adsorption into Prussian blue (PB) in order to develop high-performance PB-based Cs adsorbents in the wake of the Fukushima nuclear accident. We compared two types of PB nanoparticles with formulae of Fe
adsorbents in the wake of the Fukushima nuclear accident. We compared two types of PB nanoparticles with formulae of Fe
 [Fe
[Fe (CN)
(CN) ]3
]3 xH
xH O (x = 10-15) (PB-1) and (NH
O (x = 10-15) (PB-1) and (NH )0.70Fe
)0.70Fe 1.10[Fe
1.10[Fe (CN)
(CN) ]
] 1.7H
1.7H O (PB-2) with respect to the Cs
O (PB-2) with respect to the Cs adsorption ability. The synthesised PB-1, by a common stoichiometric aqueous reaction between 4Fe
adsorption ability. The synthesised PB-1, by a common stoichiometric aqueous reaction between 4Fe and 3[Fe
and 3[Fe (CN)
(CN) ]
] , showed much more efficient Cs
, showed much more efficient Cs adsorption ability than did the commercially available PB-2.
adsorption ability than did the commercially available PB-2.
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
Radiochimica Acta, 101(11), p.711 - 717, 2013/11
Times Cited Count:3 Percentile:25.73(Chemistry, Inorganic & Nuclear)Four new alkyl-pyridinedicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) were synthesized and used as extractants for partitioning minor actinides from high level radioactive waste using extraction chromatography. The R-PDAs were successfully impregnated into XAD resins (Amberlite). The prepared adsorbents, R-PDA/XAD, exhibited moderate adsorptions of both Am(III) and Eu(III), and separation between Am(III) and Eu(III), over a high HNO concentration range (3-5 M). Deterioration of the prepared adsorbent as a result of contact with HNO
concentration range (3-5 M). Deterioration of the prepared adsorbent as a result of contact with HNO solution was prevented by using R-PDAs containing longer alkyl groups. The equilibrium data for Eu(III) were analyzed using Langmuir and Freundlich isotherm models. The adsorption of Eu(III) ions onto the R-PDA/XAD was fitted better to the Langmuir isotherm than to the Freundlich isotherm. The maximum adsorption capacity was determined to be 24.2 mg Eu/g (R-PDA/XAD).
solution was prevented by using R-PDAs containing longer alkyl groups. The equilibrium data for Eu(III) were analyzed using Langmuir and Freundlich isotherm models. The adsorption of Eu(III) ions onto the R-PDA/XAD was fitted better to the Langmuir isotherm than to the Freundlich isotherm. The maximum adsorption capacity was determined to be 24.2 mg Eu/g (R-PDA/XAD).
Chen, R.*; Tanaka, Hisashi*; Kawamoto, Toru*; Asai, Miyuki*; Fukushima, Chikako*; Na, H.*; Kurihara, Masato*; Watanabe, Masayuki; Arisaka, Makoto; Nankawa, Takuya
Electrochimica Acta, 87, p.119 - 125, 2013/01
Times Cited Count:108 Percentile:94.92(Electrochemistry)A novel electrochemical adsorption system using a nanoparticle film of copper (II) hexacyanoferrate (III) was proposed for selectively removing cesium from wastewater. This system can be used for cesium separation without extra chemical reagents or any filtration treatment. Cesium uptake and elution can be simply controlled by switching the applied potentials between anodes and cathodes. Data from batch kinetic studies well fitted the intraparticle diffusion equation, reflecting a two-step process: a steepest ascent portion followed by a plateau extending to the equilibrium. The effective cesium removal with a high distribution coefficient (

 5
5 10
10 mL/g) can be adopted in a large pH range from 0.3 to 9.2, and in the presence of several diverse coexisting alkaline cations, suggesting it can be taken as a promising technology for actual nuclear wastewater treatment.
mL/g) can be adopted in a large pH range from 0.3 to 9.2, and in the presence of several diverse coexisting alkaline cations, suggesting it can be taken as a promising technology for actual nuclear wastewater treatment.
Chen, R.*; Tanaka, Hisashi*; Kawamoto, Toru*; Asai, Miyuki*; Fukushima, Chikako*; Kurihara, Masato*; Watanabe, Masayuki; Arisaka, Makoto; Nankawa, Takuya
Electrochemistry Communications, 25, p.23 - 25, 2012/11
Times Cited Count:50 Percentile:79.8(Electrochemistry)We first synthesized water-dispersed nanoparticle copper hexacyanoferrate (CuHCF) ink and then coated its nanoparticles on electrodes to electrochemically remove cesium from wastewater. Cesium uptake and elution can be controlled by switching the potentials between anodes and cathodes. Effective cesium removal can be adopted in a large pH range from 0.2 to 8.9, and in the presence of several diverse coexisting alkaline cations, suggesting that it can be taken as a promising technology for actual radioactive wastewater treatment. The prepared CuHCF nanoparticles can be simply and uniformly coated on electrodes by wet process like conventional printing methods, so any sizes or patterns are feasible at low cost, which indicated the potential as a promising sorption electrode of large size in the columns for sequential removal and recycle of Cs from wastewater.
Kozai, Naofumi; Onuki, Toshihiko; Arisaka, Makoto; Watanabe, Masayuki; Sakamoto, Fuminori; Yamasaki, Shinya; Jiang, M.
Journal of Nuclear Science and Technology, 49(5), p.473 - 478, 2012/05
Times Cited Count:64 Percentile:97.82(Nuclear Science & Technology)Chemical states of radioactive Cs in the contaminated soils by Fukushima Daiichi Nuclear Power Plant accident has been characterized by the desorption experiments using appropriate reagents solutions and size fractionation of the contaminated soils. Approximately 70% of radioactive Cs in the residual fraction were associated with the size fractions larger than the elutriated one, even though mica-like minerals were contained in the elutriated one. These results strongly suggest that radioactive Cs was irreversibly associated with soil components other than mica like minerals in the contaminated soil.
 -tetraoctyl-3-oxapentane-1,5-diamide in
-tetraoctyl-3-oxapentane-1,5-diamide in  -dodecane/nitric acid
-dodecane/nitric acidArisaka, Makoto; Kimura, Takaumi
Solvent Extraction and Ion Exchange, 29(1), p.72 - 85, 2011/02
Times Cited Count:48 Percentile:73.86(Chemistry, Multidisciplinary)Thermodynamic parameters (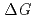 ,
, 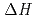 and
and 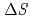 ) for the extraction of trivalent f-elements, M(III) (M = Am, Eu), with
) for the extraction of trivalent f-elements, M(III) (M = Am, Eu), with  -tetraoctyl-3-oxapentane-1,5-diamide (TODGA) were determined in nitric acid/
-tetraoctyl-3-oxapentane-1,5-diamide (TODGA) were determined in nitric acid/ -dodecane extraction system. The extraction of M(III) with TODGA was more exothermic than those with octyl(phenyl)-
-dodecane extraction system. The extraction of M(III) with TODGA was more exothermic than those with octyl(phenyl)- ,
, -diisobutylcarbamoylmethyl phosphine oxide (CMPO) and dihexyl-
-diisobutylcarbamoylmethyl phosphine oxide (CMPO) and dihexyl- ,
, -diethylcarbamoylmethyl phosphonate (DHDECMP). The difference in
-diethylcarbamoylmethyl phosphonate (DHDECMP). The difference in 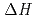 between the extractants was attributed to the difference in the binding mode between them, i.e. tridentate (TODGA) and bidentate (CMPO and DHDECMP). In addition, from the results of luminescence lifetime measurement, it was found that the inner-sphere of extracted Eu(III) was dehydrated completely, and occupied by TODGA and/or NO
between the extractants was attributed to the difference in the binding mode between them, i.e. tridentate (TODGA) and bidentate (CMPO and DHDECMP). In addition, from the results of luminescence lifetime measurement, it was found that the inner-sphere of extracted Eu(III) was dehydrated completely, and occupied by TODGA and/or NO
 .
.
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
Journal of Nuclear Materials, 407(2), p.116 - 118, 2010/12
Times Cited Count:6 Percentile:40.28(Materials Science, Multidisciplinary)Adsorption of trivalent actinides (An(III)) and lanthanides (Ln(III)) in acidic aqueous solution using activated carbons was investigated. Activated carbons exhibited significantly selective adsorption to An(III) over Ln(III) in acidic aqueous solution from about pH 1 to pH 4, independently of the starting materials, activation methods and specific surface areas.
Watanabe, Masayuki; Arisaka, Makoto; Kimura, Takaumi
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), 5 Pages, 2010/00
N,N'-dimethyl-N,N'-diphenylpyridine-2,6-dicarboxyamide which contains a pyridyl group between two amidic functional groups exhibited moderate separation ability of actinides(III) from lanthanides(III) at high HNO concentration by solvent extraction. To solve the leakage problem on the extraction chromatography, we reported that newly synthesized N,N'-dialkyl-N,N'-diphenylpyridine-2,6-dicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) exhibited reduction on the leakage of extractant from the impregnated resin depending on the length of alkyl group. The column chromatography of actinides(III) from lanthanides (III) was investigated by using the impregnated resin with Oct-PDA.
concentration by solvent extraction. To solve the leakage problem on the extraction chromatography, we reported that newly synthesized N,N'-dialkyl-N,N'-diphenylpyridine-2,6-dicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) exhibited reduction on the leakage of extractant from the impregnated resin depending on the length of alkyl group. The column chromatography of actinides(III) from lanthanides (III) was investigated by using the impregnated resin with Oct-PDA.
Watanabe, Masayuki; Arisaka, Makoto; Kimura, Takaumi
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.1062 - 1065, 2009/09
Adsorption of trivalent actinides (An(III)) and lanthanides (Ln(III)) in acidic aqueous solution with carbon nano-materials including activated carbon, graphite and carbon nanotube were investigated. These carbon nano-materials exhibited the significant selectivity to An(III) from Ln(III) in acidic aqueous solution.
 by external ultrasound irradiation
by external ultrasound irradiationToraishi, Takashi; Kimura, Takaumi; Arisaka, Makoto
Journal of Nuclear Science and Technology, 44(9), p.1220 - 1226, 2007/09
Times Cited Count:5 Percentile:37.19(Nuclear Science & Technology)An innovative remote valency control technique for actinide ions induced by external ultrasound irradiation was reported in the present study. It is known that ultrasound irradiation to water causes the oxidation and/or reduction of the solute by radicals. We very recently found that a noble metal catalyst drastically enhances the sonochemical effect, and even highly stable U(VI) is reduced to U(IV) by ultrasound irradiation. Employing this catalytic reaction, we are developing low-emission actinide ion separation schemes driven by external ultrasound irradiation. In the present work, U(VI), Np(VI) and Pu(VI) in 3 M HNO medium were chosen as target ions. Their valency was first adjusted to U(VI)/ Np(V)/ Pu(IV) by external ultrasound irradiation, and then further sonochemical reduction to U(IV)/ Np(IV)/ Pu(III) was carried out.
medium were chosen as target ions. Their valency was first adjusted to U(VI)/ Np(V)/ Pu(IV) by external ultrasound irradiation, and then further sonochemical reduction to U(IV)/ Np(IV)/ Pu(III) was carried out.
 ,
, '-dialkyl-
'-dialkyl- ,
, '-diphenylpyridine-2,6-dicarboxyamides
'-diphenylpyridine-2,6-dicarboxyamidesArisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
Proceedings of International Conference on Advanced Nuclear Fuel Cycles and Systems (Global 2007) (CD-ROM), p.111 - 113, 2007/09
Four  ,
, '-dialkyl-
'-dialkyl- ,
, '-diphenylpyridine-2,6-dicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) were newly synthesized and were applied to extraction chromatography as extractant to attain the separation of actinides(III) from high level radioactive waste containing lanthanides(III). R-PDA was successfully impregnated into XAD-4 resin. It was found that (i) the leakage of R-PDA from XAD-4 resin was suppressed with an increase of the length of the alkyl groups in R-PDA, while the leakage for each adsorbent resin was promoted with an increase of HNO
'-diphenylpyridine-2,6-dicarboxyamides (R-PDA; R = butyl, octyl, decyl, dodecyl) were newly synthesized and were applied to extraction chromatography as extractant to attain the separation of actinides(III) from high level radioactive waste containing lanthanides(III). R-PDA was successfully impregnated into XAD-4 resin. It was found that (i) the leakage of R-PDA from XAD-4 resin was suppressed with an increase of the length of the alkyl groups in R-PDA, while the leakage for each adsorbent resin was promoted with an increase of HNO concentration in the aqueous phase and (ii) Oc-PDA or De-PDA/XAD-4 resin exhibits moderate separation ability of actinides(III) from lanthanides(III) at relatively high HNO
concentration in the aqueous phase and (ii) Oc-PDA or De-PDA/XAD-4 resin exhibits moderate separation ability of actinides(III) from lanthanides(III) at relatively high HNO concentration.
concentration.
Nagaishi, Ryuji; Arisaka, Makoto; Kimura, Takaumi; Kitatsuji, Yoshihiro
Journal of Alloys and Compounds, 431(1-2), p.221 - 225, 2007/04
Times Cited Count:44 Percentile:86.38(Chemistry, Physical)In order to elucidate coordination states of europium(III) ion in ionic liquids (ILs) and its physicochemical behavior, spectroscopic and electrochemical properties of Eu(III) were studied as a function of water content in hydrophobic ILs consisting of anion (bis(trifluoromethanesulfonyl)imide = tfsi) and cation (imidazolium or ammonium). The luminescence studies indicate that Eu(III) acts spectroscopically as aqua complex in the water-saturated ILs giving 9 of the inner-sphere hydration number of Eu(III), and that at the hydration number  9 tfsi anion involves in asymmetry of the coordination sphere of Eu(III) and in the quenching of photo-excited Eu(III). The electrochemical studies show that the divalent Eu(II) is more stable in the IL than in aqueous solution, and that the motion of Eu(III) is inhibited effectively by the matrix of IL.
9 tfsi anion involves in asymmetry of the coordination sphere of Eu(III) and in the quenching of photo-excited Eu(III). The electrochemical studies show that the divalent Eu(II) is more stable in the IL than in aqueous solution, and that the motion of Eu(III) is inhibited effectively by the matrix of IL.
Sasaki, Yuji; Rapold, P.*; Arisaka, Makoto; Hirata, Masaru; Kimura, Takaumi; Hill, C.*; Cote, G.*
Solvent Extraction and Ion Exchange, 25(2), p.187 - 204, 2007/03
Times Cited Count:136 Percentile:92.69(Chemistry, Multidisciplinary)Extraction of Eu(III) and Am(III) from HNO into the organic solvents using N,N,N',N'-tetraoctyl-diglycolamide (TODGA) was investigated in order to study the detailed extraction reaction. The chemical species: 1:2 for metal:TODGA complex is present in polar diluents. On the other hand, the metal complexes need three or more number of TODGA molecules to remain stable in non-polar diluents. The HNO
into the organic solvents using N,N,N',N'-tetraoctyl-diglycolamide (TODGA) was investigated in order to study the detailed extraction reaction. The chemical species: 1:2 for metal:TODGA complex is present in polar diluents. On the other hand, the metal complexes need three or more number of TODGA molecules to remain stable in non-polar diluents. The HNO concentration dependence on the distribution ratio suggests that HNO
concentration dependence on the distribution ratio suggests that HNO participates in the metal extraction. Infrared spectra indicate that the carbonyl oxygen coordinates with Eu(III), and luminescence lifetimes suggest that there is no water molecule in the inner coordination sphere of Eu-complex extracted.
participates in the metal extraction. Infrared spectra indicate that the carbonyl oxygen coordinates with Eu(III), and luminescence lifetimes suggest that there is no water molecule in the inner coordination sphere of Eu-complex extracted.
Toraishi, Takashi; Kimura, Takaumi; Arisaka, Makoto
Chemical Communications, (3), p.240 - 241, 2007/01
Times Cited Count:8 Percentile:34.99(Chemistry, Multidisciplinary)We here report the enhancement of a sonochemical effect (chemical reaction induced by ultrasound irradiation) by a Pt black catalyst; the sonochemical reduction of the highly stable U(VI) was demonstrated using this catalytic reaction.
Arisaka, Makoto; Kimura, Takaumi; Nagaishi, Ryuji; Yoshida, Zenko
Journal of Alloys and Compounds, 408-412, p.1307 - 1311, 2006/02
Times Cited Count:6 Percentile:42.74(Chemistry, Physical)no abstracts in English